CBSE Class-12 Chemistry
Quick Revision Notes
Chapter 13
Amines
• Amines: Amines are regarded as derivatives of ammonia in which one, two or all
three hydrogen atoms are replaced by alkyl or aryl group.
• Classification of amines:
(i) By reduction of nitro compounds: Nitro compounds can be catalytically reduced by
passing hydrogen gas in presence of Raney Ni, finely divided Pt or Pd as catalyst at room
temperature.
Ni,Pt or pd
a)
Ni, Pt or pd
b)
Nitro compounds can also be reduced with active metals such as Fe, Sn, Zn etc. with conc.
HCl.
Sn/HCl or Fe/HCl
a)
Sn/HCl or Fe/HCl
- Ar — NO2 + 3.¾ >■ Ar — iVfl2 + 2.¾ 0
(ii) By Hoffmann’s method (Ammonolysis of alkyl halides): Reaction of alkyl halides with
an ethanolic solution of ammonia in a sealed tube at 373 K forms a mixture of primary,
secondary and tertiary amine and finally quarternary ammonium salt. Process of cleavage of
C-X bond by ammonia is called ammonolysis.
+■ —
RNH2 — > R2NH f–l: > R3N K>: > S4 A7 X
(£) (2e) (je) Q*at9T#nrj/.
3Tjy.PT.LUTL E-L |[ I
- The free amine can be obtained from the ammonium salt by treatment with a strong
base:
NaOH
a)
(l°a min e)
NaOH
b)
(2°amine)
NaOH
c)
(3° a min e)
- Order of reactivity of halides is: RI>RBr>RCl
- Larger the size of halogen atom easier is the cleavage of R-X bond
- Limitations of Hoffmann’s method: Method gives mixture of amines which are
difficult to separate in a laboratory. - Methods to get only one product by Hoffmann’s method:
- When ammonia is taken in excess primary amine is formed as main product
- When alkyl halide is used in excess quarternary ammonium salt is formed as main
product.
Method is not suitable for preparation of aryl amines because aryl amines are relatively less
reactive than alkyl halides towards nucleophilic substitution reactions.
- By reduction of nitriles: Nitriles can be reduced to amines using H2 / Ni , LiAlH4 or
Na(Hg) / C2H5 OH
H2/Ni
Or
Na(Hg)/C2H5OH
Or
LiAlHt
- By reduction of amides: Amides are reduced to corresponding amines by LiAlH4
- By Gabriel phthalimide synthesis: Gabriel synthesis is used for the preparation of
primary amines. When phthalimide is treated with ethanolic potassium hydroxide, it forms
potassium salt of phthalimide which on heating further with alkyl halide followed by
alkaline hydrolysis produces the corresponding primary amine.
Aromatic primary amines cannot be prepared by this method because aryl halides do not
undergo nucleophilic substitution with potassium phthalimide.
- By Hoffmann bromamide degradation reaction: Primary amines can be prepared from
amides by treatment with Br2 and KOH. Amine contains one carbon atom less than the
parent amide.
0
Il
i
R – NH2 + Na2 CO2 + 2 NaBr + 2 H2O
Physical properties of amines:
- Solubility: Lower aliphatic amine is soluble in water because they can form hydrogen
bonding with water. Solubility decreases with increases in molar mass of amines due to
increase in size of hydrophobic group - Boiling points: Among the isomeric amines primary and secondary amines have high
boiling point because they can form hydrogen bonding. Tertiary amine cannot form
hydrogen bonding due to the absence of hydrogen atom available for hydrogen bond
formation. Hence order of boiling of isomeric amines is Primary>Secondary> Tertiary
• Chemical properties of amines:
- Basic character of amines: Amines have an unshared pair of electrons on nitrogen atom
due to which they behave as Lewis base. Basic character of amines can be better understood
in terms of their Kb and pKb values
[.R-NHz][OH]
[R-NH2][H20\
[R-NHs][OH]
Or K[H20] =
[R-NH2]
_ [R-NH3][OH]
b [R-NH2]
pKb = -Iogif6
Greater Kb value or smaller pKb indicates base is strong.
- Comparison of basic strength of aliphatic amines and ammonia: Aliphatic amines are
stronger bases than ammonia due to +I effect of alkyl groups leading to high electron density
on the nitrogen atom. - Comparison of basic strength of primary, secondary and tertiary amines
(i) The order of basicity of amines in the gaseous phase follows the expected order on the
basis of +I effect: tertiary amine > secondary amine > primary amine > NH3
(ii) In aqueous solution it is observed that tertiary amines are less basic than either primary
or secondary amines. This can be explained on basis of following factors:
- Solvation effect: Greater is the stability of the substituted ammonium cation formed,
stronger is the corresponding amine as a base. Tertiary ammonium ion is less hydrated than
secondary ammonium ion which is less hydrated than primary amine. Thus tertiary amines
have fewer tendencies to form ammonium ion and consequently are least basic.
On the basis of solvation effect order of basicity of aliphatic amines should be primary
amine>secondary amine>tertiary amine.
- Steric factor: As the crowding of alkyl group increases from primary to tertiary amine
hinderance to hydrogen bonding increases which eventually decreases the basic strength.
Thus there is a subtle interplay of the inductive effect, solvation effect and steric hinderance
of the alkyl group which decides the basic strength of alkyl amines in the aqueous state.
When the alkyl group is small like CH3 there is no steric hindrance to hydrogen bonding. In
this case order of basicity in aqueous medium is
(CH3)2NH > CH3NH2 > (CH3)3N > NH3
When alkyl group is ethyl group order of basicity in aqueous medium is
(C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3
- Comparison of basic strength of aryl amines and alkylamines: Generally aryl amines are
considerably less basic than alkyl amines .Taking an example of aniline and ethylamine it is
observed that ethyl amine is more basic than aniline. In aniline -NH2 group is directly
attached to benzene ring. Hence unshared pair of electron on nitrogen is less available for
protonation because of resonance. Below mentioned are resonating structures of aniline.
In the above resonating structures there is a positive charge on nitrogen atom making the
lone pair less available for protonation. Hence aniline is less basic than ethyl amine which
has no resonating structures. Less basicity of aniline can also be explained by comparing the
relative stability of aniline and anilinium ion obtained by accepting a proton. Greater the
number of resonating structures, greater is the stability of that species.
Aniline is resonance hybrid of five resonating structures whereas anilinium ion has only two
resonating structures.
Thus aniline has less tendency to accept a proton to form anilinium ion.
- Effect of substituent on basic character of amines: Electron donating or electron releasing
group/groups (EDG) increases basic strength while electron withdrawing (EWG) decreases
basic strength.
a) Acylation Reaction: Aliphatic and aromatic primary and secondary amines (which
contain replaceable hydrogen atoms) react with acid chlorides, anhydrides and esters to
form substituted amide. Process of introducing an acyl group (R-CO-) into the molecule is
called acylation. The reaction is carried out in the presence of a stronger base than the
amine, like pyridine, which removes HCl formed and shifts the equilibrium to the product
side.
Base
R – NH2 + RCOCl > RNHCOR + HCl
Add chloride Substituted amide
^Li bzmwed em^
Since tertiary amine do not contain replaceable hydrogen atom they do not undergo
acylation reaction.
b) Carbylamine reaction: Only aliphatic and aromatic primary amines on heating with
chloroform and ethanolic potassium hydroxide form isocyanides or carbylamines.
R – NH0 + CHCl + 3 KOH
2. .-
|
1 F
R – NC + 3KCl + ^H2O
Secondary and tertiary amines do not give the above test.
(i) Primary aliphatic amine on reaction with nitrous acid (HNO2) forms aliphatic
diazoniumsalt which decomposes to form alcohol and evolve nitrogen.
(ii) Primary aromatic amines react with nitrous acid (HNO2) in cold (273-278 K) to form
diazonium salt.
- Reaction with benzene sulphonyl chloride: Hinsberg’s reagent-Benzenesulphonyl chloride
(C6H5SO2Cl) reacts with primary and secondary amines to form sulphonamides.
The hydrogen attached to nitrogen in sulphonamide formed by primary amine is strongly
acidic due to the presence of strong electron withdrawing sulphonyl group. Hence, it is
soluble in alkali.
Since sulphonamide formed by secondary amine does not contain any hydrogen atom
attached to nitrogen atom, so it is not acidic. Hence it is insoluble in alkali.
• Ring substitution in aromatic amine: Aniline is more reactive than benzeneand
undergoes electrophilic substitution reaction preferably at ortho and para position.
(i) Bromination: Aniline reacts with bromine water at room temperature to give a white
precipitate of 2, 4, 6-tribromoaniline
In order to stop reaction at monosubstitution activating effect of -NH2 group is reduced by
acetylation. This prevents di and tri substituted products. Acetyl group is removed by
hydrolysis.
(a) Under strongly acidic medium aniline gets protonated to form anilinium ion, which is
deactivating group and is meta directing. Hence minitroaniline is also formed in 47% along
with ortho and para products.
NOj
(51¾) (47%) (2%)
Aromatic amines cannot be nitrated directly because HNO3 being a strong oxidising agent
oxidises it forming black mass.
(b) Nitration by protecting the -NH2 group by acetylation reaction with acetic anhydride:
iii) Sulphonation: Aniline reacts with conc. H2SO4 to form aniliniumhydrogensulphate which
on heating with sulphuric acid at 453-473K produces p-aminobenzenesulphonic acid,
commonly known as sulphanilic acid, as the major product.
• Reactions ofbenzene diazonium chloride:
a) Reactions involving displacement of nitrogen:
Material Downloaded From SUPERCOP
/ 11
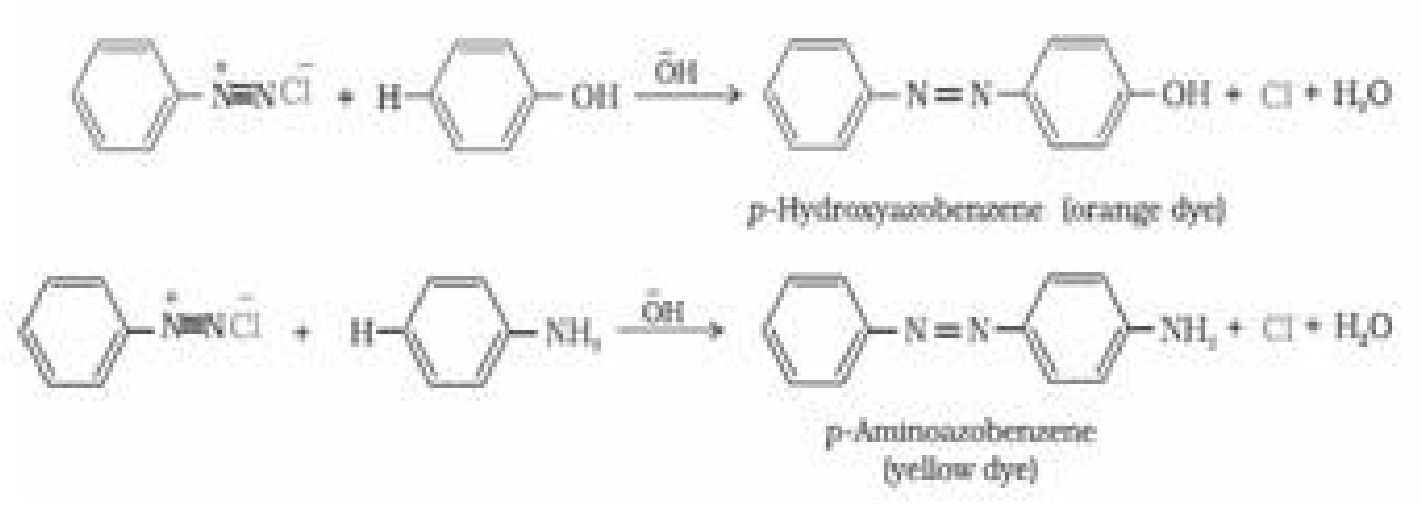
b) Reactions involving retention of diazo group, coupling reactions: Diazonium ion
acts as an electrophile because there is a positive charge on terminal nitrogen.
Therefore benzene diazonium chloride couples with electron rich compounds like
phenol and aniline to give azo compounds. Azo compounds contain -N=N- bond and
reaction is coupling reaction.