CSBE Class 12 Chemistry
Revision Notes
Chapter 12
Aldehydes, Ketones and Carboxylic acid
Aldehydes: Aldehydes are the organic compounds in which carbonyl group is attached to
one hydrogen atom and one alkyl or aryl group.

Where R can be an alkyl or aryl group
- By oxidation of alcohols: Oxidation of primary alcohols in presence of oxidizing agent like
K2Cr2O7/H2SO4, KMnO4,CrO3 gives aldehydes.

- By dehydrogenation of alcohols: When the vapours of primary alcohol passed through
heated copper at 573 K, it forms aldehyde.
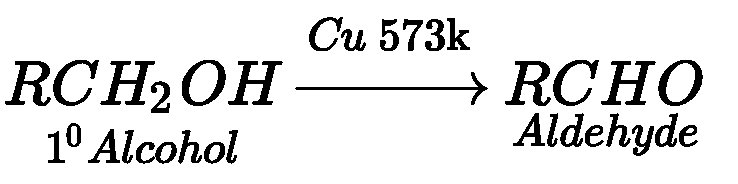
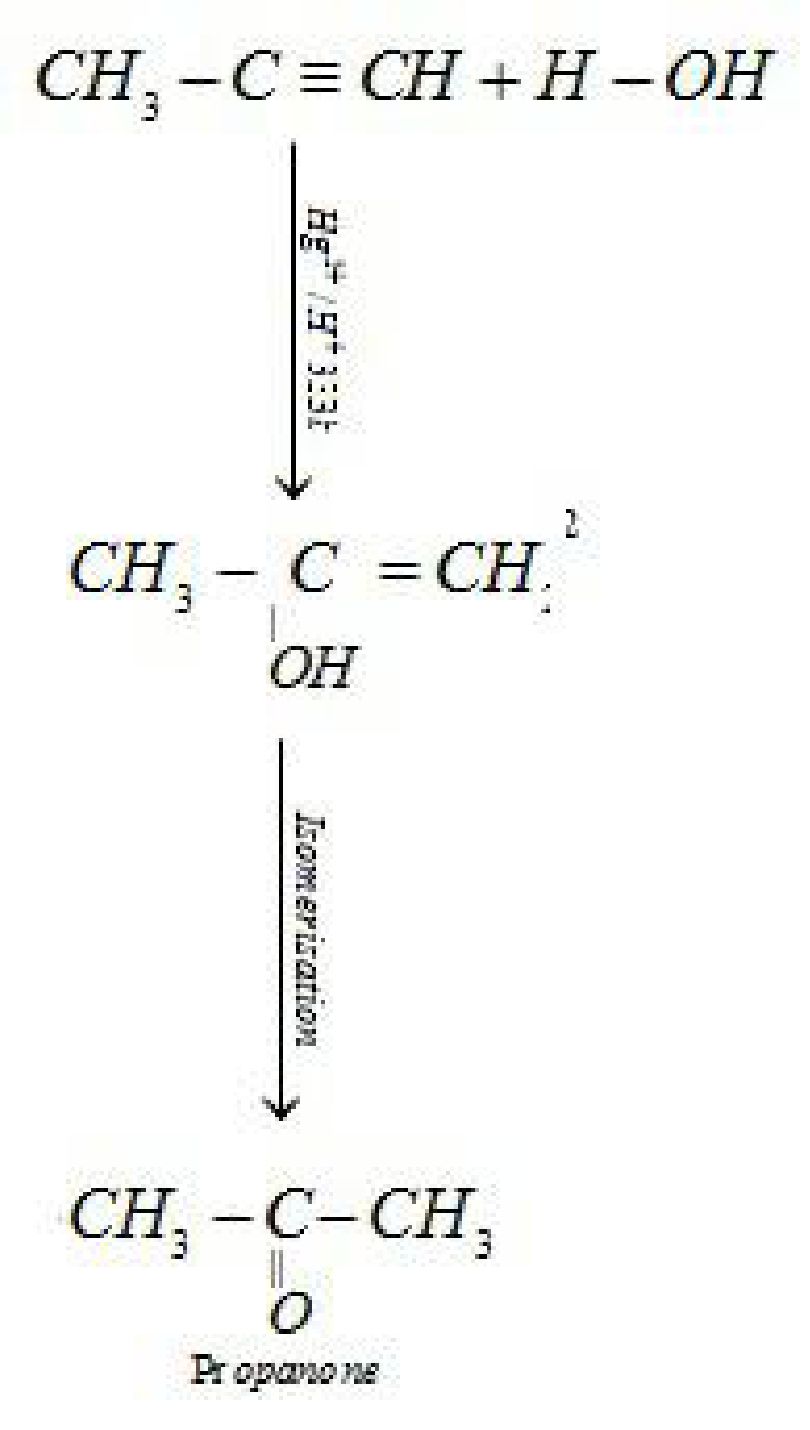
- By hydration of alkynes: Ethyne on hydration withat 333 K forms
acetaldehyde.
- By reduction of nitriles:

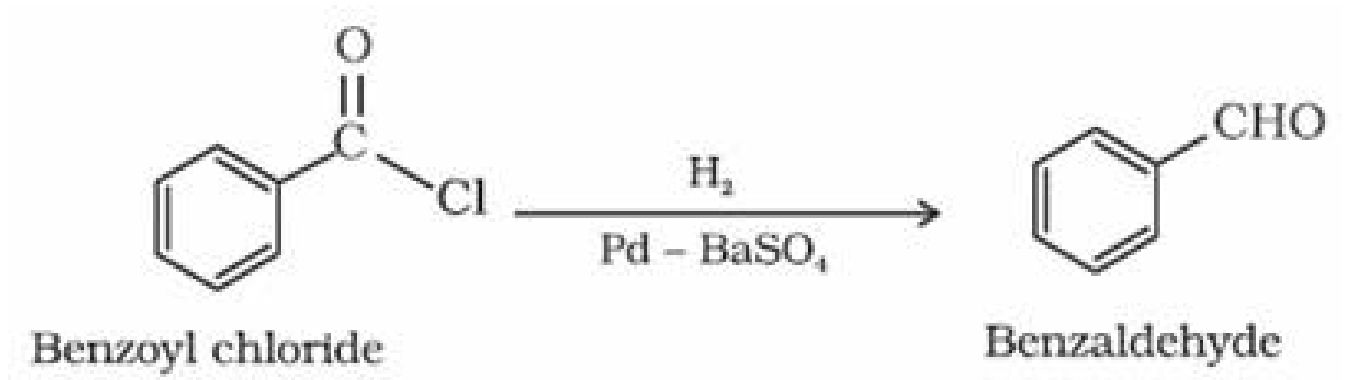
d) By Rosenmund reduction: Hydrogenation of acyl chloride over palladium on barium
sulphate gives aldehyde.
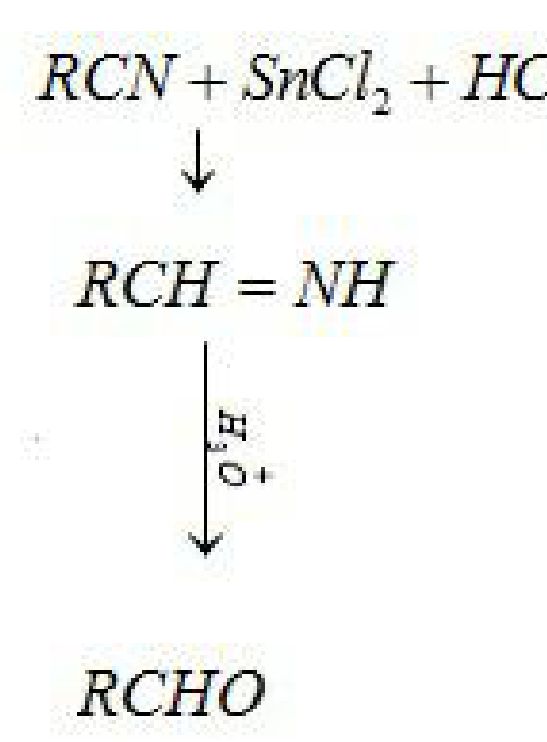
i) Stephen Reaction: Reduction of nitriles in presence of stannous chloride in presence of HCl
gives imine which on hydrolysis gives corresponding aldehyde.
ii) Nitriles are selectively reduced by DIBAL-H (Diisobutylaluminium hydride) to aldehydes.
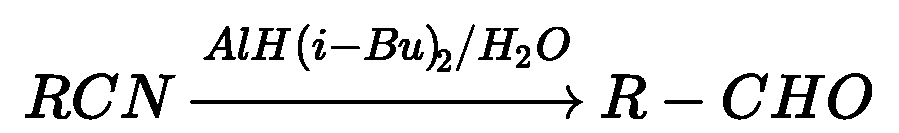
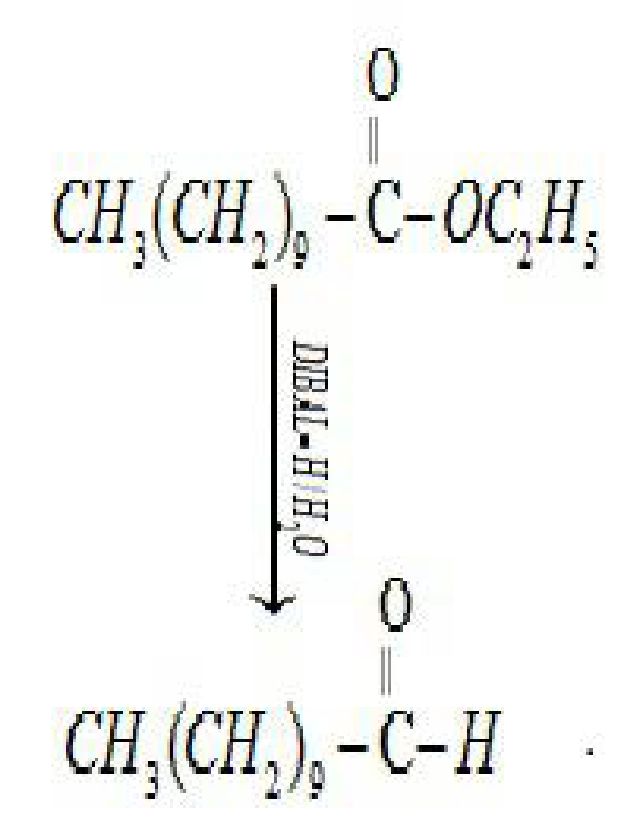
- By reduction of ester: Esters are reduced to aldehydes in presence of DIBAL-H
(Diisobutylaluminium hydride)
- From Hydrocarbons:
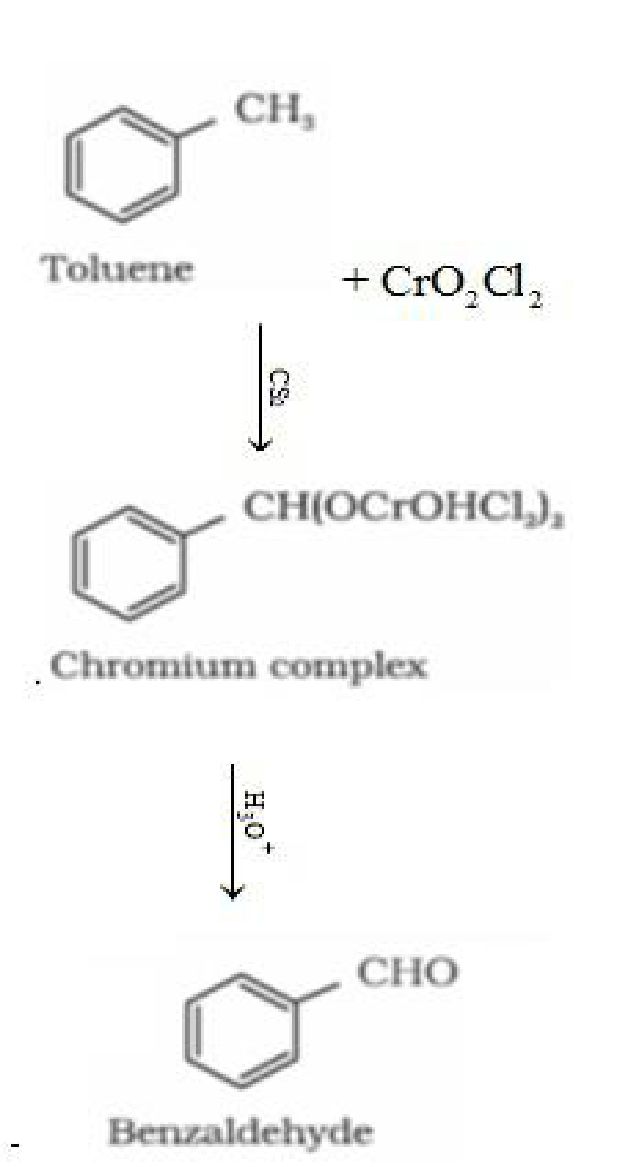
- By oxidation of methyl benzene: Etard Reaction: Chromyl chloride {CrO2Cl2) oxidizes
methyl group to a chromium complex, which on hydrolysis gives corresponding
benzaldehyde.
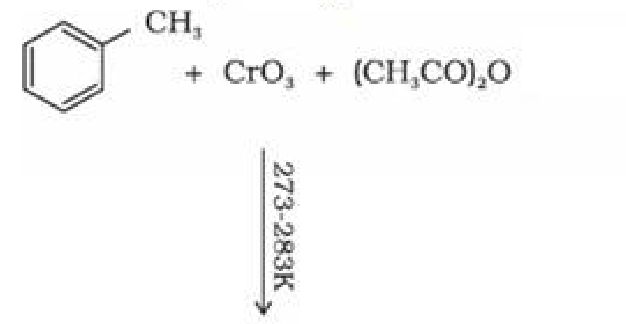
Using chromium oxide(Cr03): Toluene or substituted toluene is converted to benzaldehyde
in presence of chromic oxide in acetic anhydride.

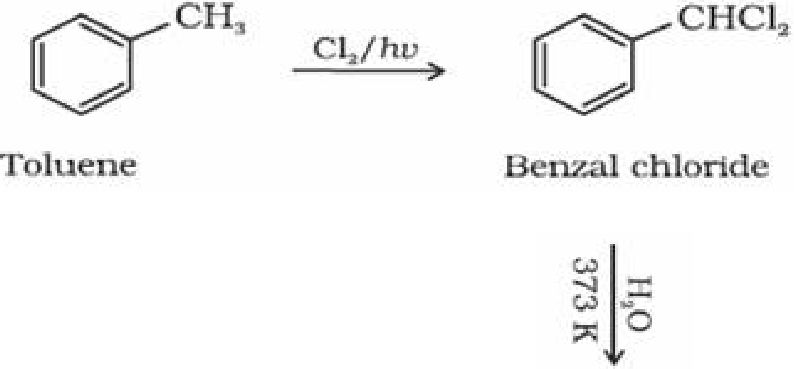
- By side chain chlorination followed by hydrolysis:Halogenation of toluene: Side chain
halogenation of toluene gives benzal chloride which on hydrolysis gives Benzaldehyde.
- Gatterman -Koch reaction: Benzene or its derivatives on treatment with carbon
monoxide and HCl in presence of anhydrous aluminium chloride or cuprous chloride (CuCl)
gives benzaldehyde or substituted benzaldehydes.

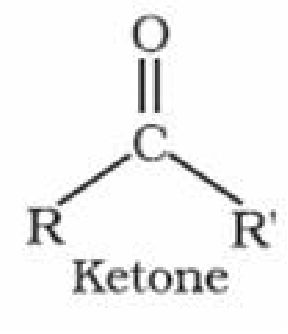
Ketones: Ketones are the organic compounds in which carbonyl group is attached to
two alkyl group or aryl group or both alkyl and aryl group.

- From acyl chloride: Acyl chloride on treatment with dialkyl cadmium (prepared by
reaction of cadmium chloride with Grignard reagent) gives ketone.
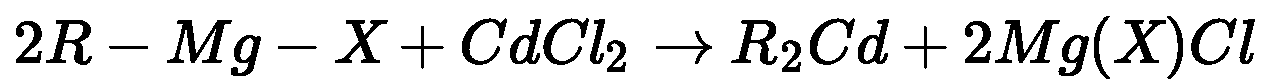

- From nitriles: Nitriles on treatment with Grignard reagent followed by hydrolysis give
ketones.

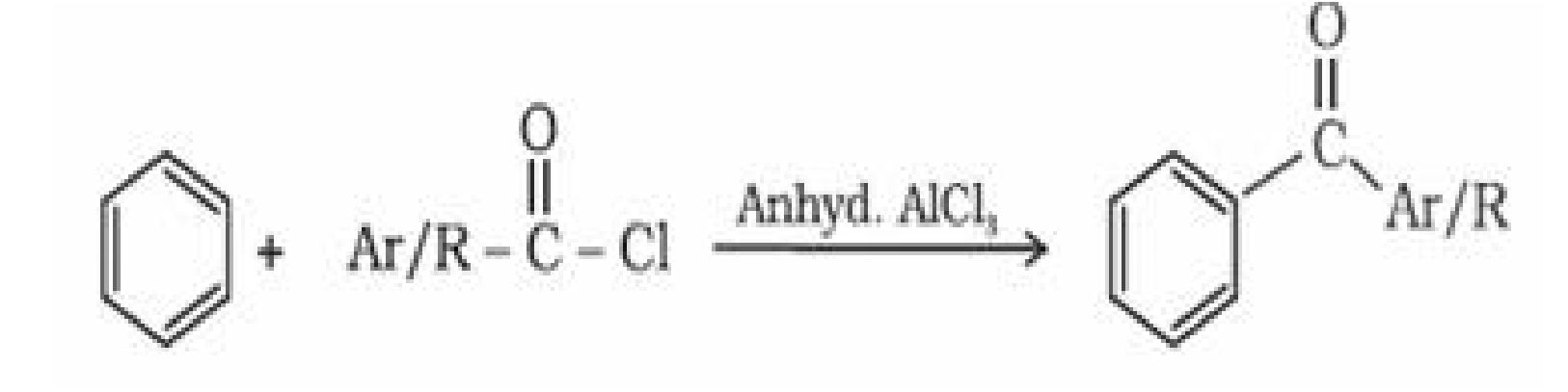
- By Friedel Crafts acylation reaction: Benzene or substituted benzene on treatment with
acid chloride in presence of anhydrous aluminium chloride forms ketone. - Preparation of aldehydes and ketones by ozonolysis of alkenes:
l I
C=C —
Propene
+ O1– ^ —C C —
O — O
| Zn + I ^O
— C = O + O = C —
Ald&bydes orKetones
- Aldehydes are generally more reactive than ketones in nucleophilic addition reactions
due to steric and electronic reasons (or inductive effect). - Electronic Effect: Relative reactivities of aldehydes and ketones in nucleophilic addition
reactions is due the positive charge on carbonyl carbon. Greater positive charge means
greater reactivity. Electron releasing power of two alkyl groups in ketones is more than
one in aldehyde. Therefore positive charge is reduced in ketones as compared to
aldehydes. Thus ketones are less reactive than aldehydes. - Stearic Effect: As the number and size of alkyl group increase, the hindrance to the attack
of nucleophile also increases and reactivity decreases. In aldehydes there is one alkyl
group and one hydrogen atom, whereas in ketones there are two alkyl groups (same or
different).
(a) Addition of hydrogen cyanide (HCN) to form cyanohydrins
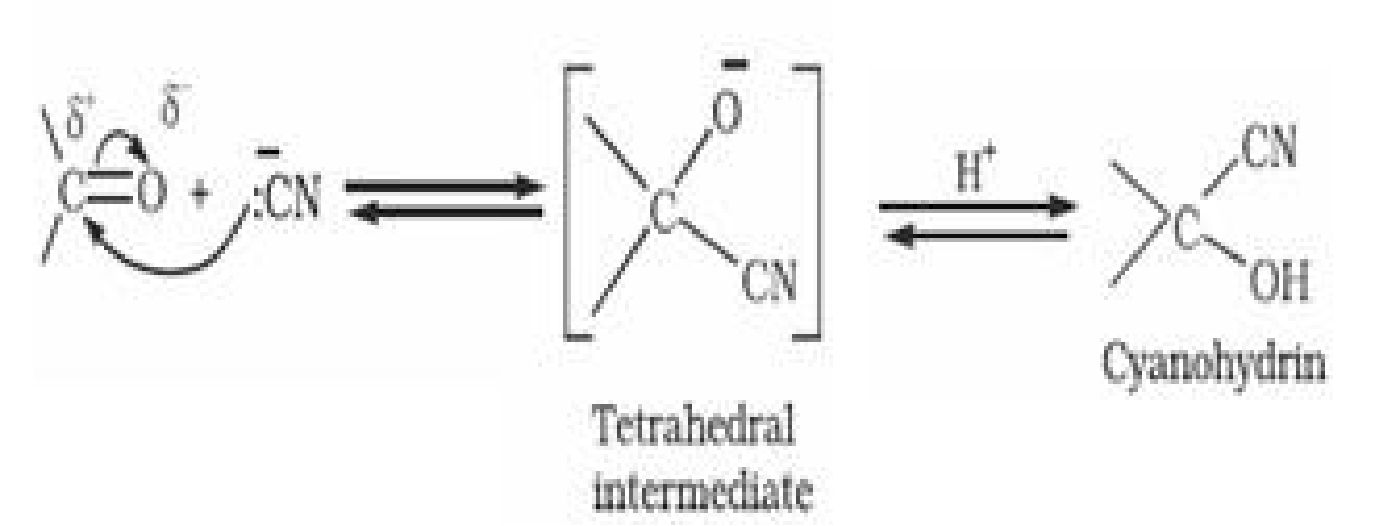
(b) Addition of sodium hydrogensulphite(^a#,L>O3)to form bisulphate addition compound

(c) Addition of Grignard reagent (RMgX) to form alcohol
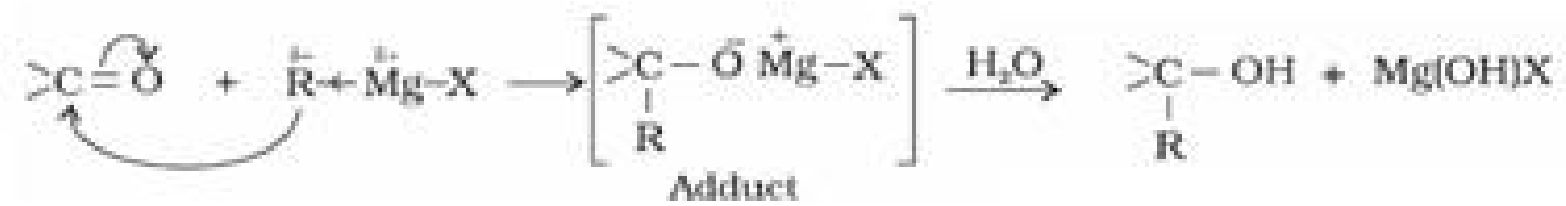
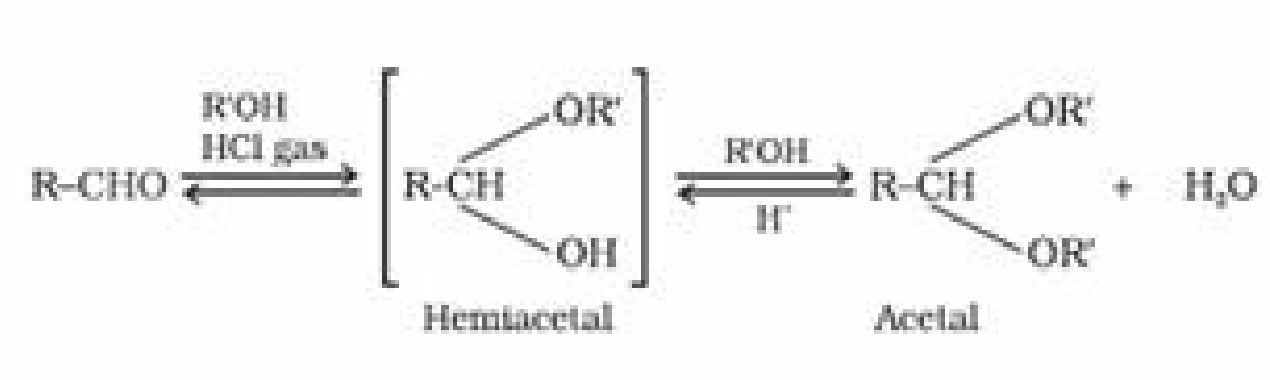
(d) Addition of alcohol:
- Aldehydes on addition of monohydric alcohol in presence of dry HCl forms hemiacetal
and acetal.
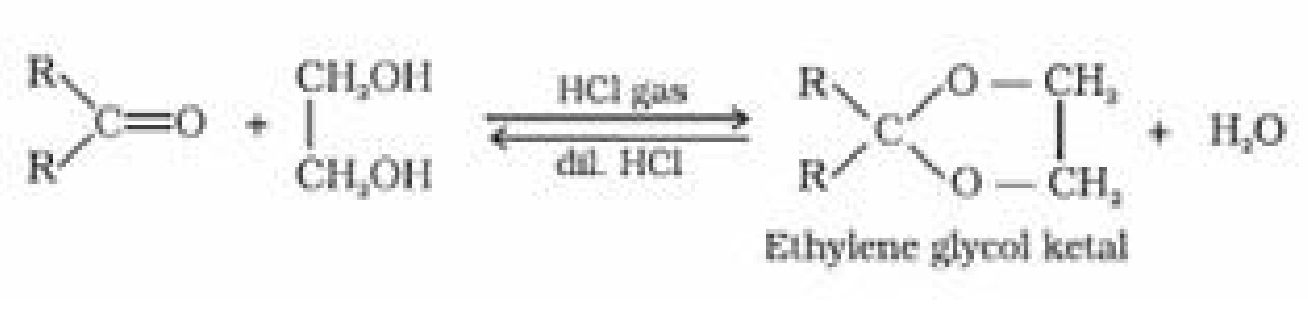
- Ketones do not react with monohydric alcohols. Ketones react with ethylene glycol under
similar conditions to form cyclic products known as ethylene glycol ketals.
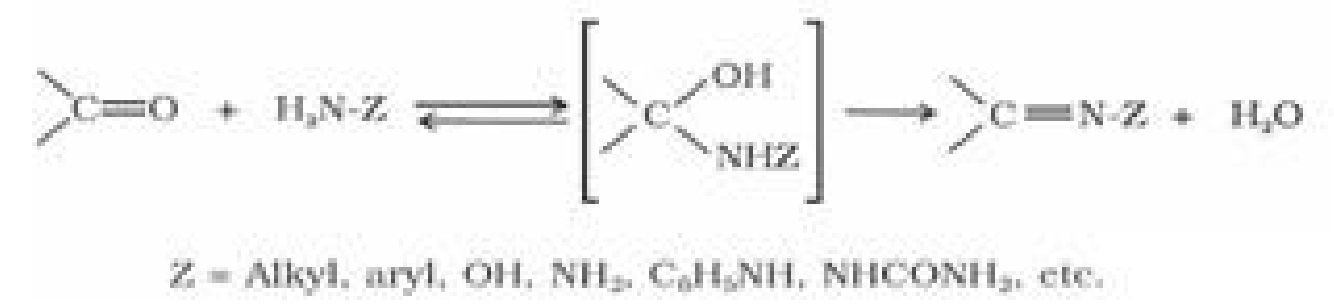
(e) Addition of ammonia and its derivatives:
Reduction of aldehydes and ketones:
(a) Reduction to alcohols:
Aldehydes and ketones on catalytic hydrogenation in presence of Ni, Pt or Pd by using
lithium aluminium hydride {LiAlH4) or sodium borohydride (NaBH4) forms primary
and secondary alcohols respectively.
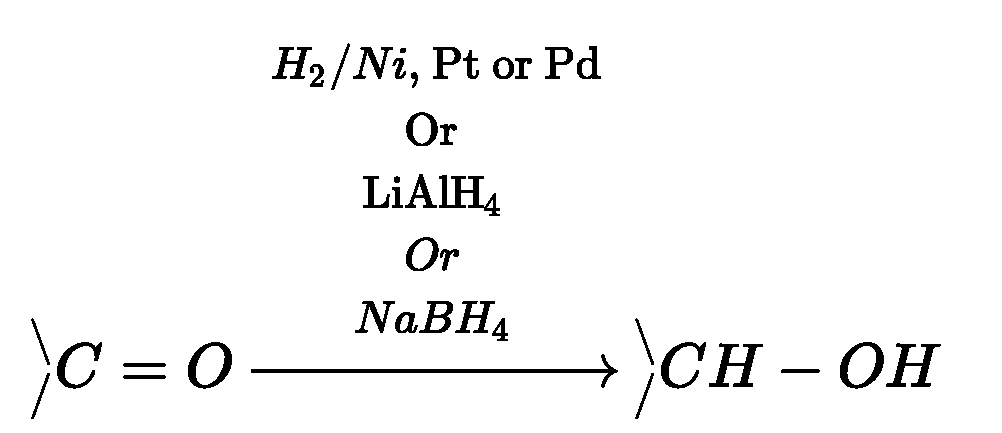
(b) Reduction to hydrocarbons:
- Clemmensen reduction: Carbonyl group of aldehydes and ketones is reduced to CH^
group on treatment with zinc amalgam and concentrated hydrochloric acid.
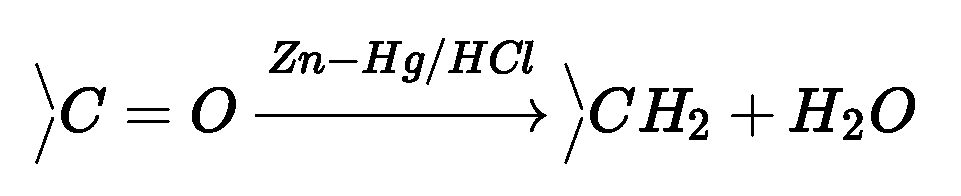
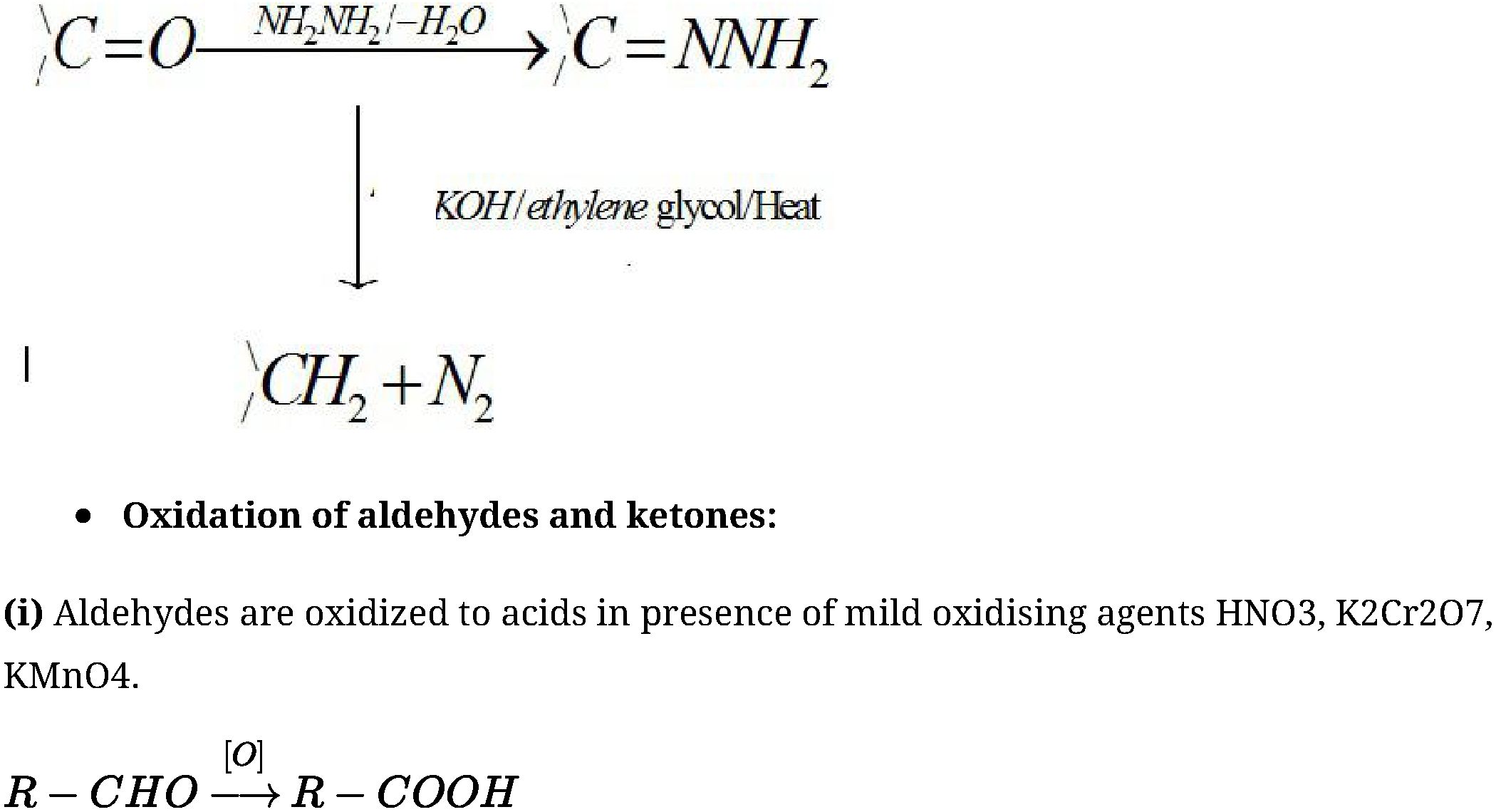
- Wolff-Kishner reduction: Carbonyl group of aldehydes and ketones is reduced to CH^
group on treatment with hydrazine followed by heating with sodium or potassium hydroxide
in high boiling solvent such as ethylene glycol.
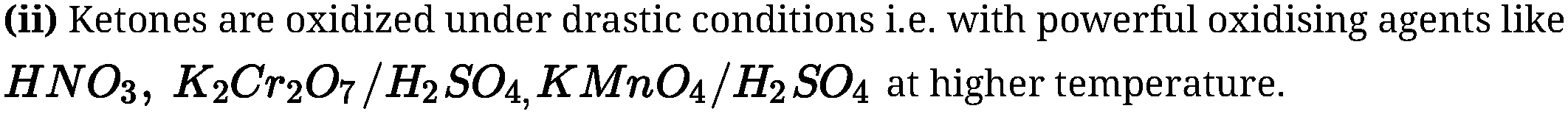
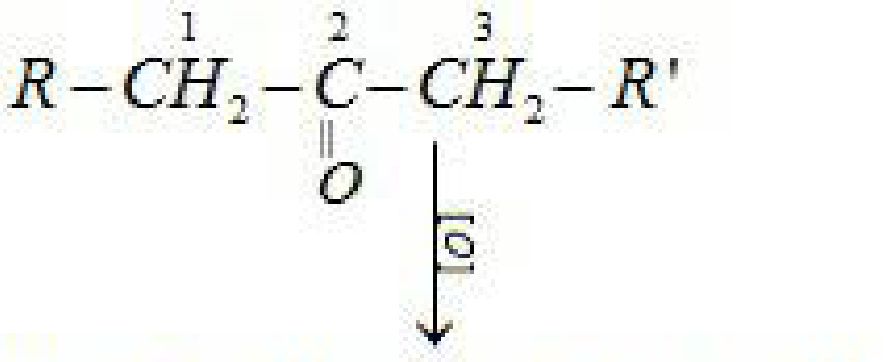
R-COOHH- J? -CHzCOOH
(By dfav^e OiCt-C^wif)
+
(j5T ■: lMV15* of Cj – Cjiw*J)
In case of unsymmetrical ketones cleavage occurs in such a way that keto group stays with
smaller alkyl group. This is known as Popoffs rule.
- Haloform reaction: Aldehydes and ketones having at least one methyl group linked to the
carbonyl carbon atom i.e. methyl ketones are oxidised by sodium hypohalite to sodium salts
of corresponding carboxylic acids having one carbon atom less than that of carbonyl
compound. The methyl group is converted to haloform.
• Reactions of aldehydes and ketones due to a -hydrogen:
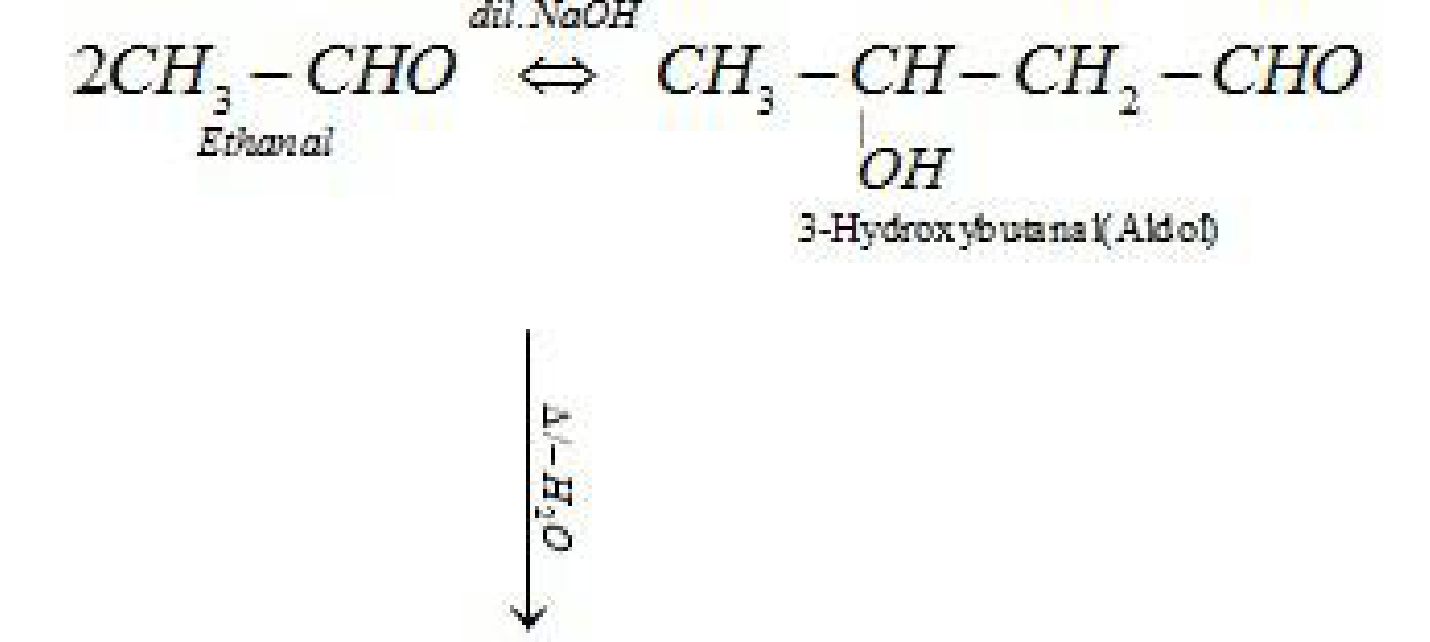
- Aldol condensation: Aldehydes and ketones having at least one a -hydrogen undergo a
self condensation in the presence of dilute alkali as catalyst to form a -hydroxy aldehydes
(aldol) or a -hydroxy ketones (ketol), respectively.
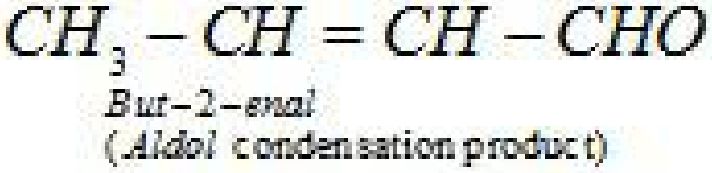
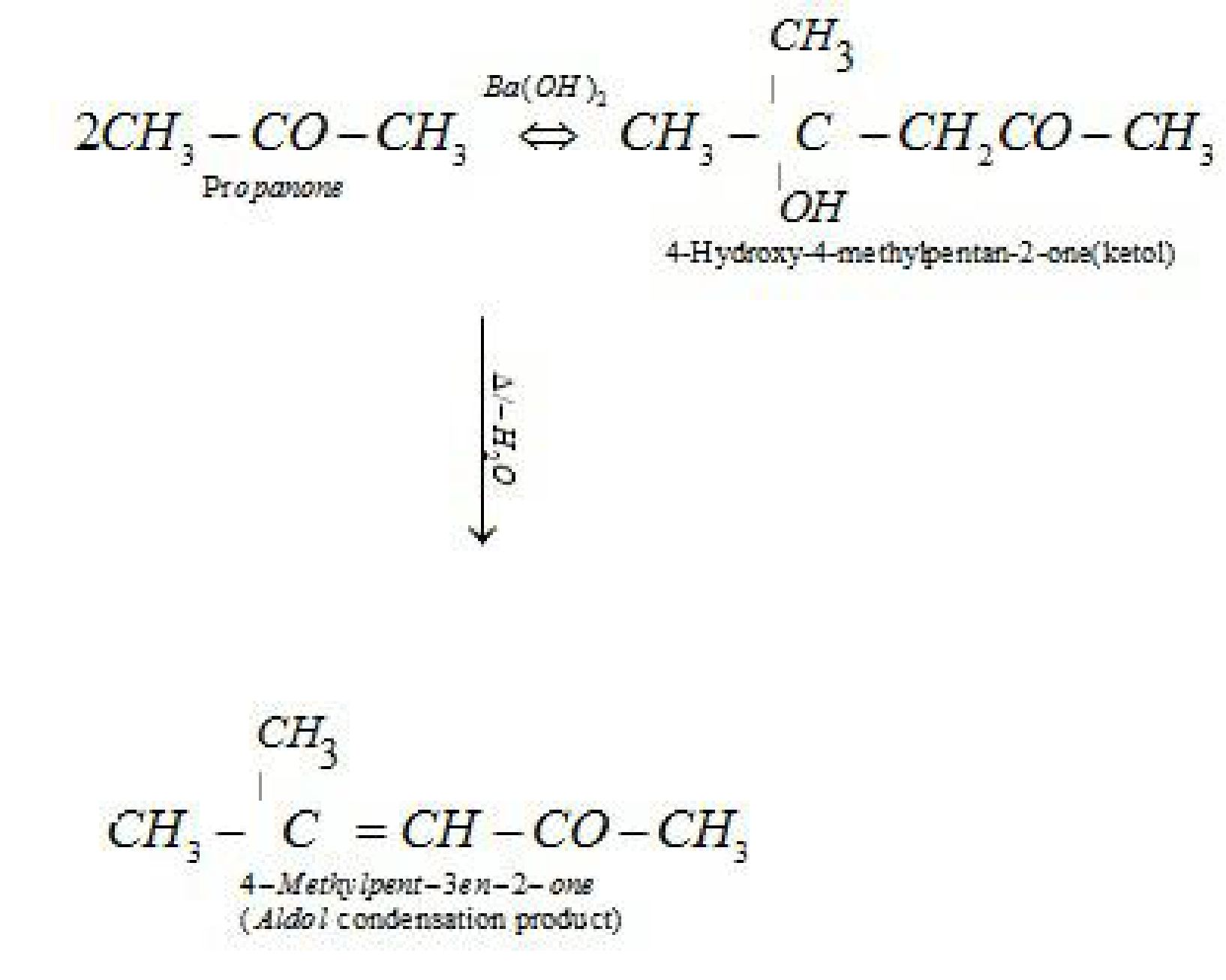
- Cross aldol condensation: Aldol condensation between two different aldehydes and
ketones is called aldol condensation. If both of them contain a -hydrogen atoms, it gives a
mixture of four products. [1]
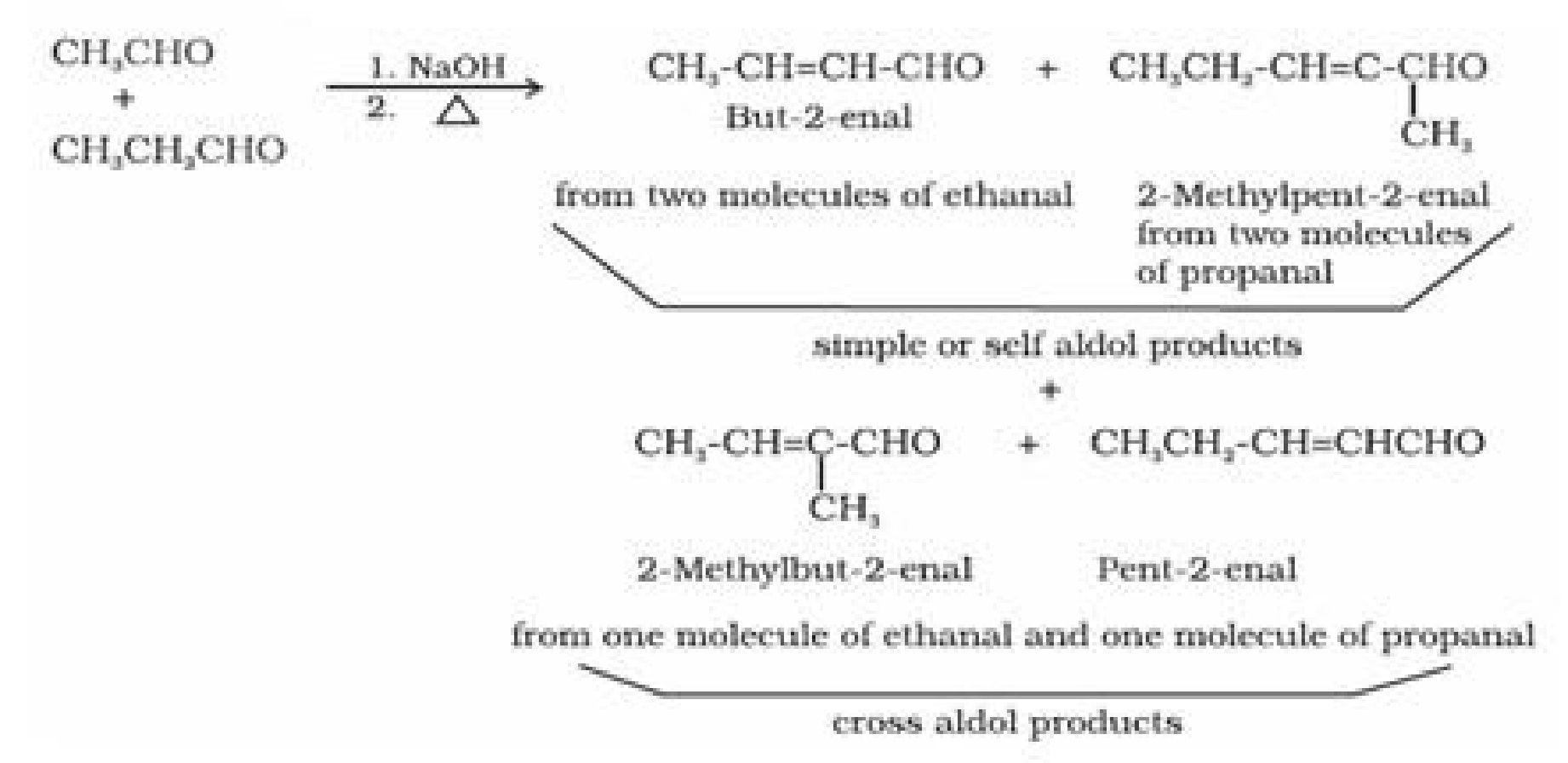
• Test to distinguish aldehydes and ketones:
- Tollen’s test: When an aldehyde is heated with Tollen’s reagent it forms silver mirror.
Tollen’s reagent is ammoniacal solution of AgNO3
Mi&+%j£m3)/+3ar ^Rcvai-24g+2H,o+4XH,
Ketones do not form silver mirror and hence do not give this test.
- Fehling’s test: When an aldehyde is heated with Fehling’s reagent it formsreddish brown
precipitates of cuprous oxide.Fehling’s reagent: Fehling solution A (aqueous solution of
CuSO4) + Fehling solution B (alkaline solution of sodium potassium tartarate)
R-CH0+2Cu1++50H~^RC00-+ Cu2O +3 H2O
Kfc^-&n*n ppl
Ketones do not give this test.
• Carboxylic Acids:Carboxylic acids are the compounds containing the
carboxylfunctional group (-COOH).
0

• Preparation of carboxylic acid:
(i) From alcohols: Primary alcohols are readily oxidised to carboxylic acids with common
oxidising agents such as potassium permanganate {KMnO4) in neutral, acidic or alkaline
media or by potassium dichromate (K2Cr2O7) and chromium trioxide (CrO3) in acidic
media.
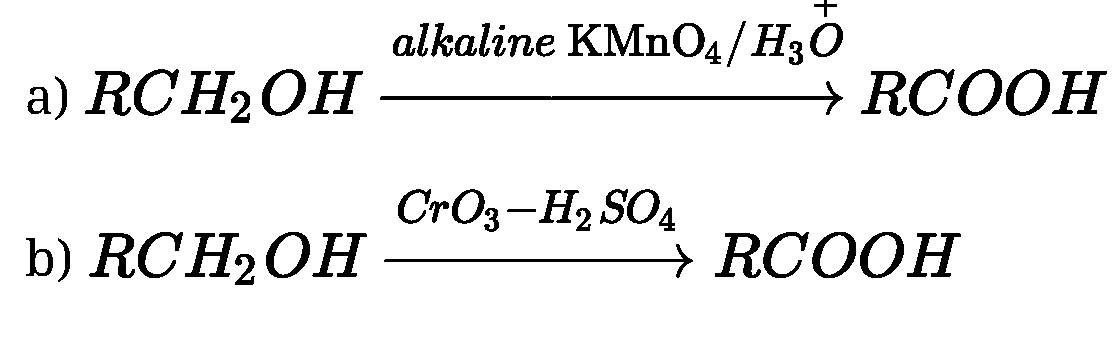
- From aldehydes: Oxidation of aldehydes in presence of mild oxidizing agents like Tollen’s
reagent (ammoniacal solution of AgNOs) or Fehling reagent (Fehling solution A (aqueous
solution of CuSO4) + Fehling solution B (aqueous solution of sodium potassium tartarate))
forms carboxylic acids.
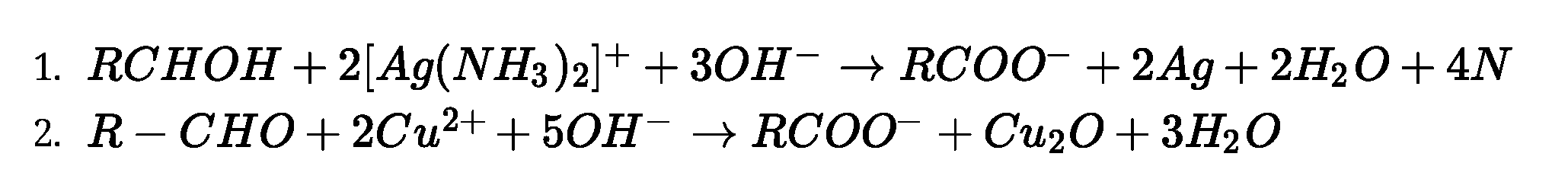
- From alkylbenzenes: Aromatic carboxylic acids can be prepared by vigorous oxidation of
alkyl benzenes with chromic acid or acidic or alkaline potassium permanganate.
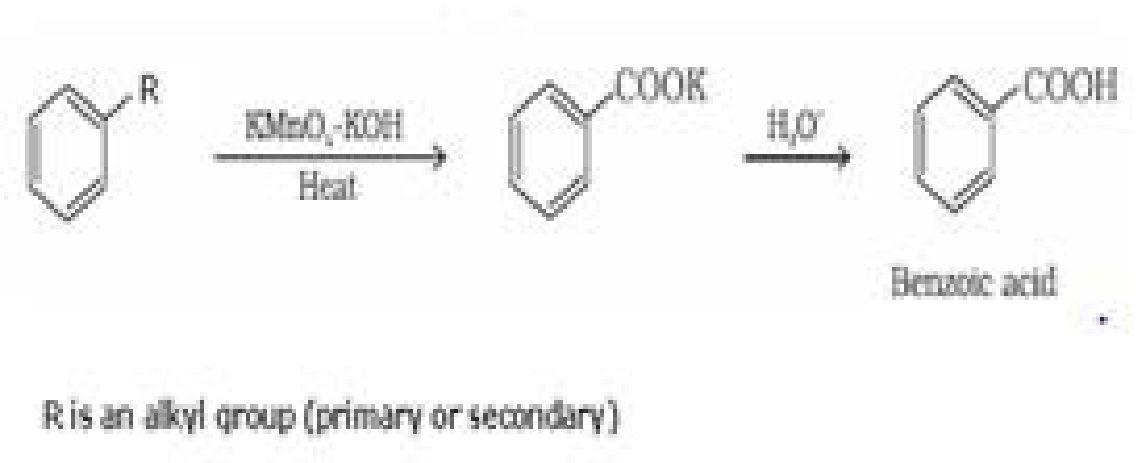
- From alkenes: Suitably substituted alkenes are oxidised to carboxylic acids on oxidation
with acidic potassium permanganate or acidic potassium dichromate.

- From Nitriles: Nitriles on hydrolysis in presence of dilute acids or bases forms amide
which on further hydrolysis gives carboxylic acid.
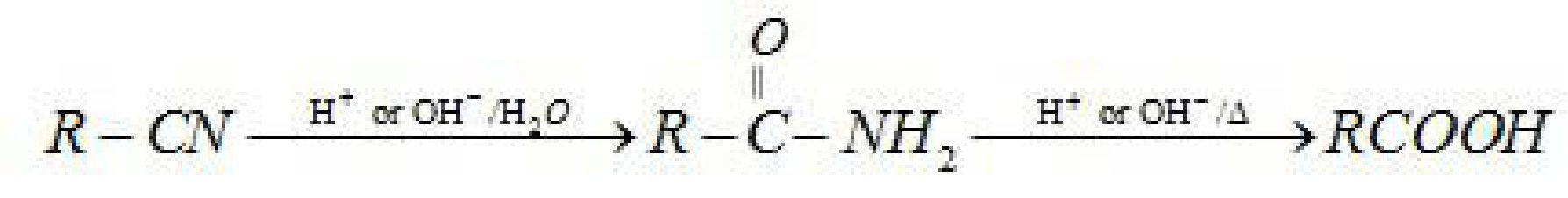
- From Grignard reagent: Grignard reagents react with carbon dioxide (dry ice) to form
salts of carboxylic acids which on hydrolysis forms carboxylic acids.
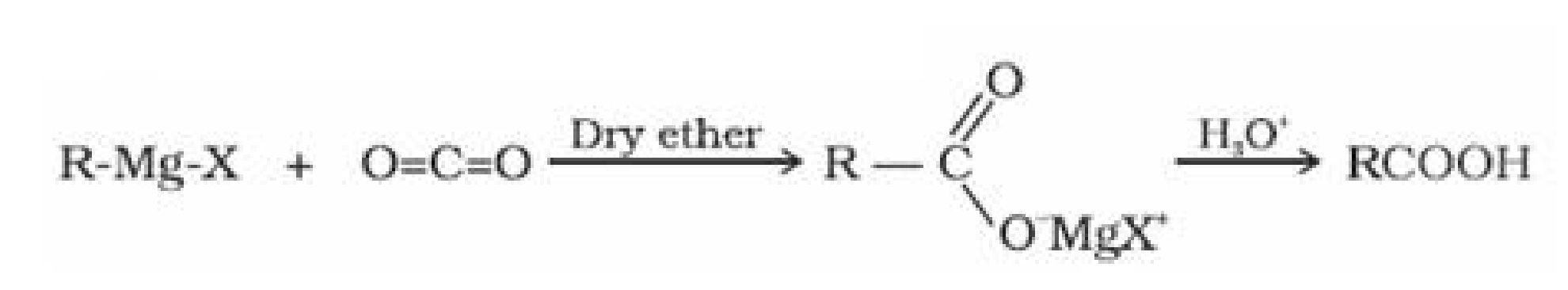
- From acyl halides and anhydrides: Acid chlorides when hydrolysed with water give
carboxylic acids .On basic hydrolysis carboxylate ions are formed which on further
acidification forms corresponding carboxylic acids. Anhydrides on hydrolysis forms
corresponding acid(s)
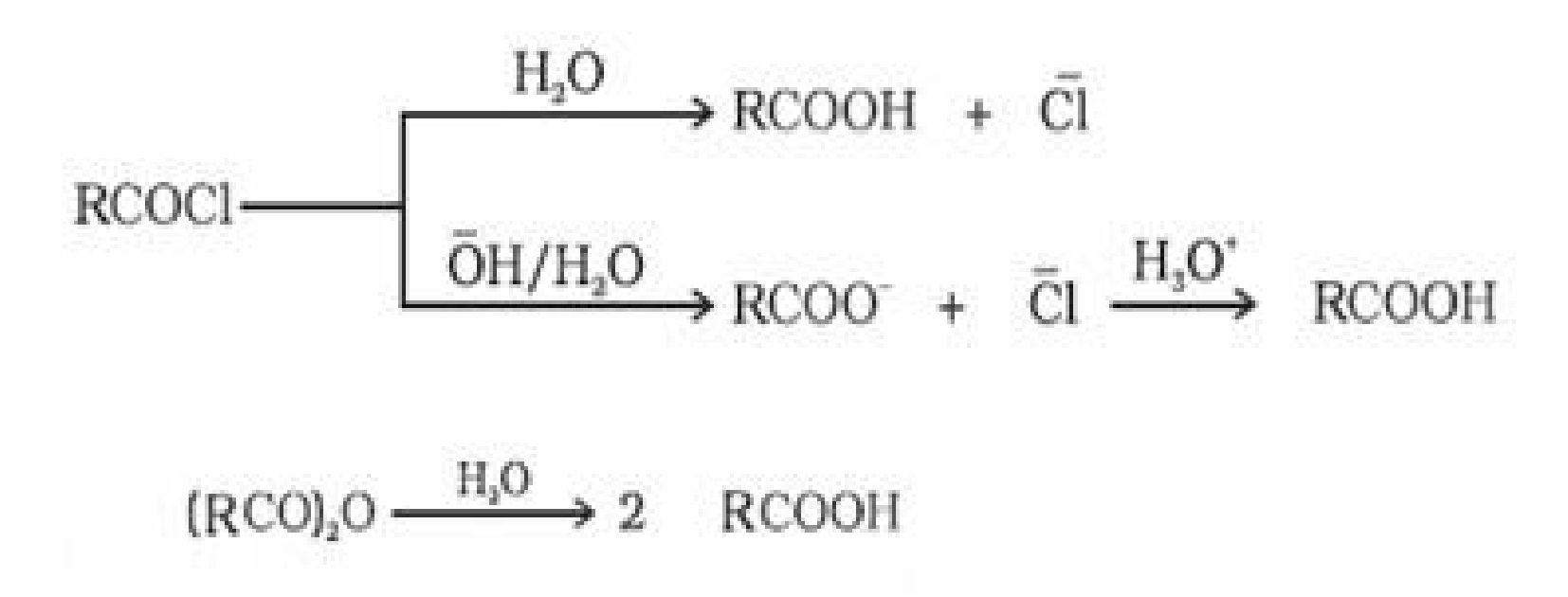
- From esters: Acidic hydrolysis of esters gives directly carboxylic acids while basic
hydrolysis gives carboxylates, which on acidification give corresponding carboxylic acids.
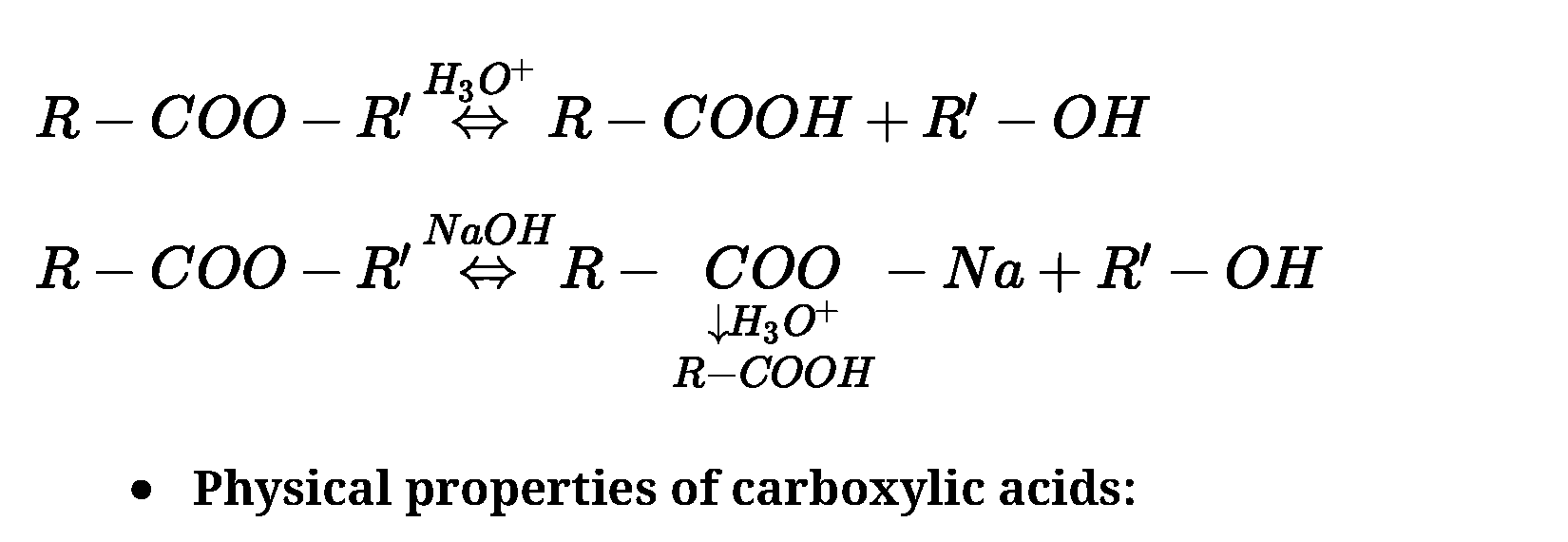
- Solubility: As the size of alky group increases solubility of carboxylic acid decreases
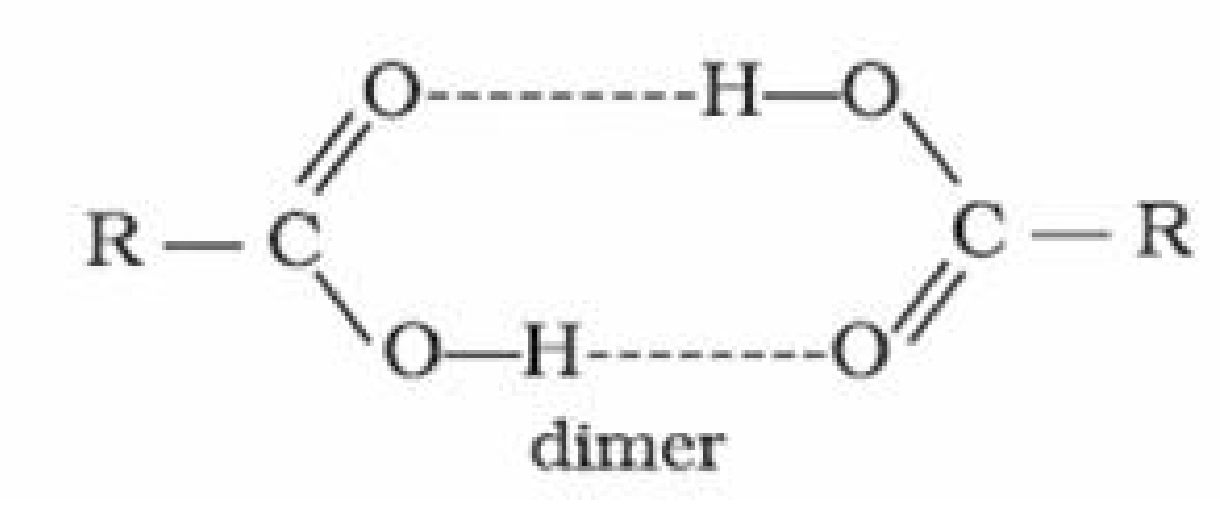
because non-polar part of the acid increases - Boiling points: Carboxylic acids are higher boiling liquids than aldehydes, ketones and
even alcohols of comparable molecular masses. This is due to extensive association of
carboxylic acid molecules through intermolecular hydrogen bonding.
• Acidity of carboxylic acids:
Carboxylic acids are more acidic than phenols. The strength of acid depends on extent of
ionization which in turn depends on stability of anion formed.
- Effect of electron donating substituents on the acidity of carboxylic acids: Electron
donating substituent decreases stability of carboxylate ion by intensifying the negative
charge and hence decreases acidity of carboxylic acids. - Effect of electron withdrawing substituent on the acidity of carboxylic acids: Electron
withdrawing group increases the stability of carboxylate ion by delocalizing negative charge
and hence, increases acidity of carboxylic acid. The effect of the following groups in
increasing acidity order is Ph< I < Br 2 < CF3 - Effect of number of electron withdrawing groups: As the number of electron withdrawing
groups increases -I effect increases, increasing the acid strength - Effect of position of electron withdrawing group: As the distance between electron
withdrawing group and carboxylic group increases, electron withdrawing influence
decreases.
• Reaction of carboxylic acids:
Reactions involving cleavage of C-OH bond:
Carboxylic acids on heating with mineral acids such as H2SO4 or with P2O5 give
corresponding anhydride.
(i) Anhydride formation:

(ii) Esterification: Carboxylic acids are esterified with alcohols in the presence of a mineral
acid such as concentrated H2SO4 or HCl gas as a catalyst.

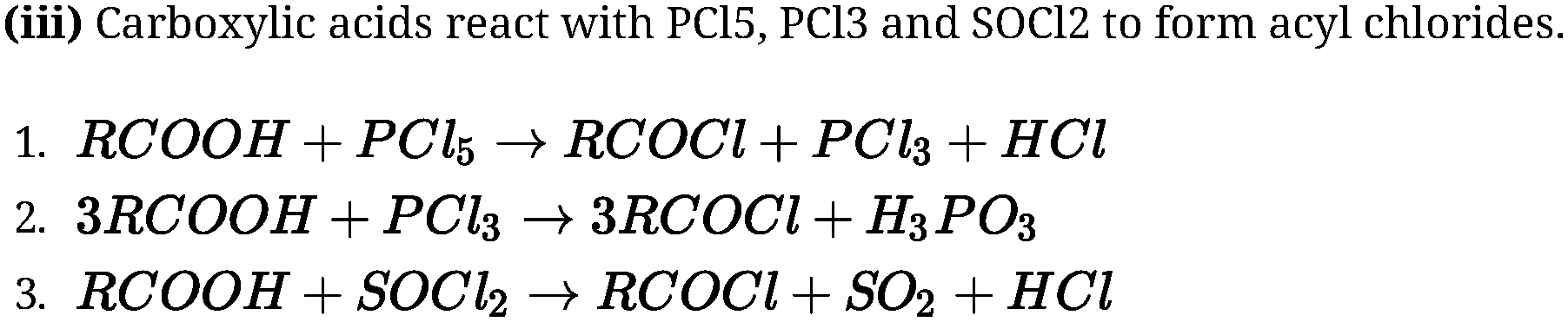

Auirnoniuin benzoatc Lfcnutmidc
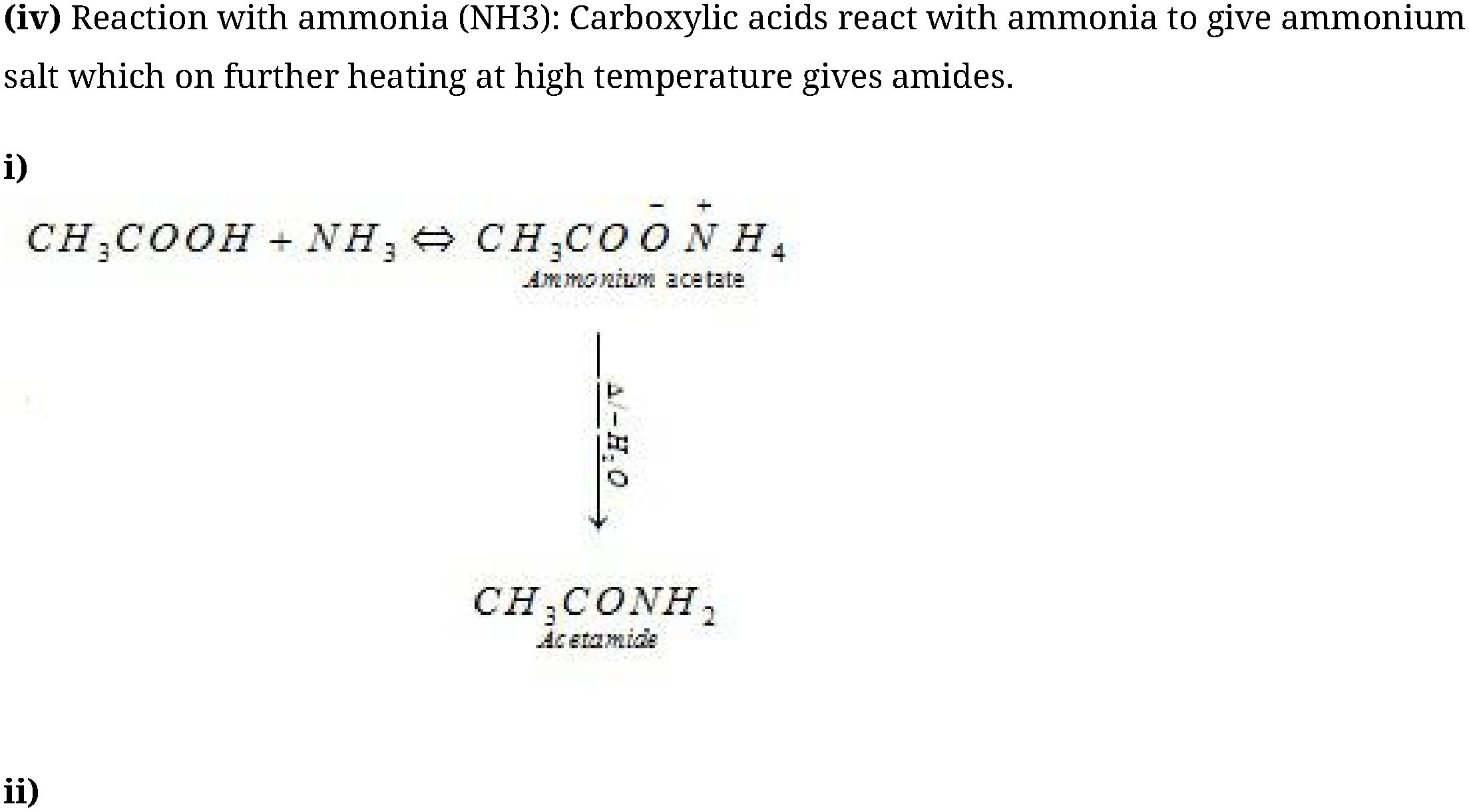
Reactions involving COOH group:
- Reduction: Carboxylic acids are reduced to alcohols in presence of LiAlH4 or B2H6.

- Decarboxylation : Sodium or potassium salts of carboxylic acids on heating with soda
lime (NaOH + CaO in ratio of 3:1) gives hydrocarbons which contain one carbon less than the
parent acid.
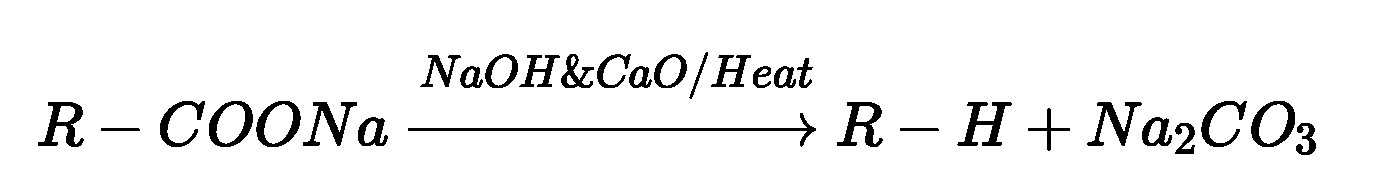
- Reactions involving substitution reaction in hydrocarbon part:
(i) Hell-Volhard-Zelinsky reaction: Carboxylic acids having an ct-hydrogen are halogenated
at the a-position on treatment with chlorine or bromine in the presence of small amount of
red phosphorus to give a-halocarboxylic acids)
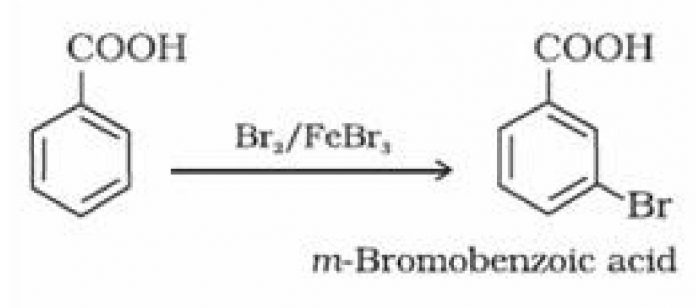
(ii) Ring substitution in aromatic acids: Aromatic carboxylic acids undergo electrophilic
substitution reactions. Carboxyl group in benzoic acid is electron withdrawing group and is
meta directing.
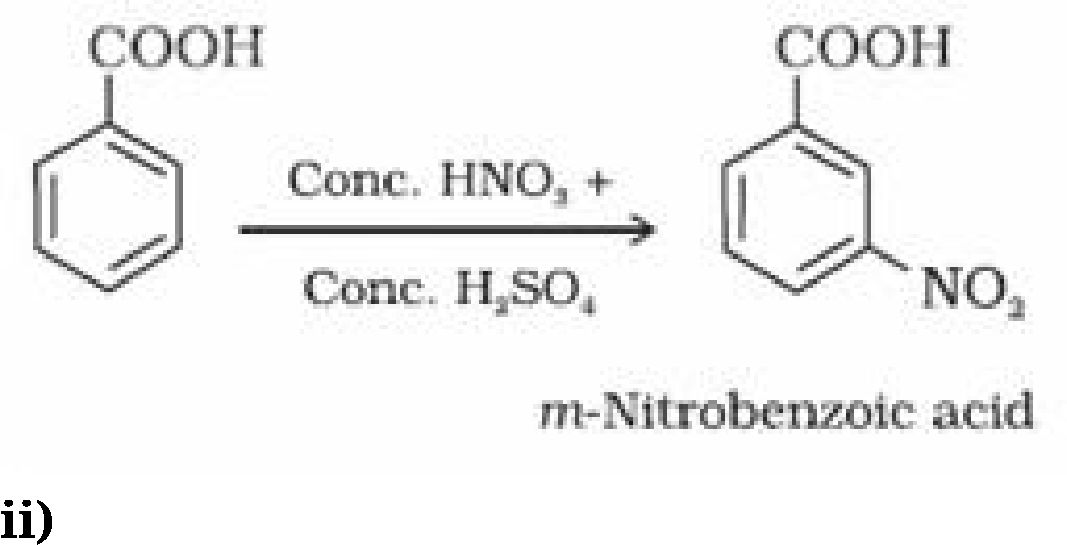
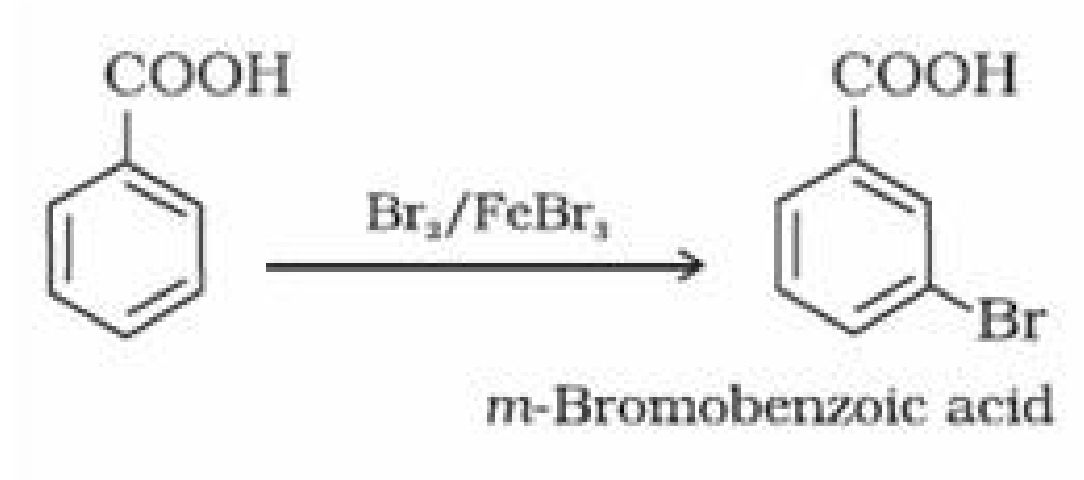
-
Canizzaro reaction: Aldehydes which do not have an a. -hydrogen atom undergo
self-oxidation and reduction (disproportionation) reaction on treatment with
concentrated alkali to form alcohol and salt of acid. ↑