CBSE Class-12 Chemistry
Quick Revision Notes
Chapter 14
Biomolecules
- Carbohydrates: Polyhydroxy aldehydes or polyhydroxyketones or compounds on
hydrolysis give carbohydrates. - Classification of carbohydrates:
Monosaccharides
- Simplest carbohydrates
- It cannot be hydrolysed into simpler compounds
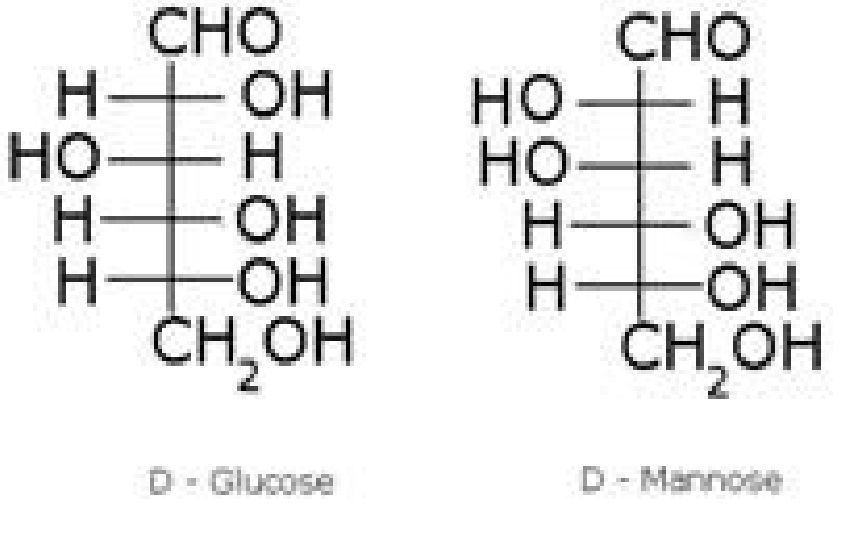
- Examples – Glucose, mannose
Oligosaccharides - Carbohydrates which gives 2 to 10 monosaccharide units on hydrolysis
- Examples – Sucrose, Lactose, Maltose
Polysaccharides - Carbohydrates which on hydrolysis give large number of monosaccharide units.
- Examples – Cellulose, starch
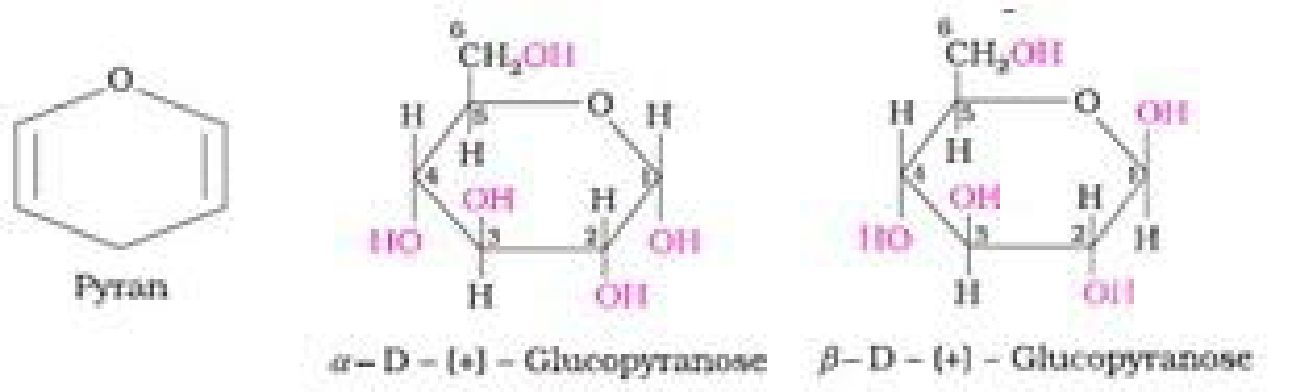
- Anomers: Pair of optical isomers which differ in configuration only around C1 atom
are called anomers. Examples – c*-D-glucopyranose and /3-D-glucopyranose. - Epimers: Pair of optical isomers which differ in configurationaround any other C
atom other than C1 atom are called epimers. E.g. D-glucose and D- mannose are
C2epimers.
Preparation of glucose (also called dextrose, grape sugar):
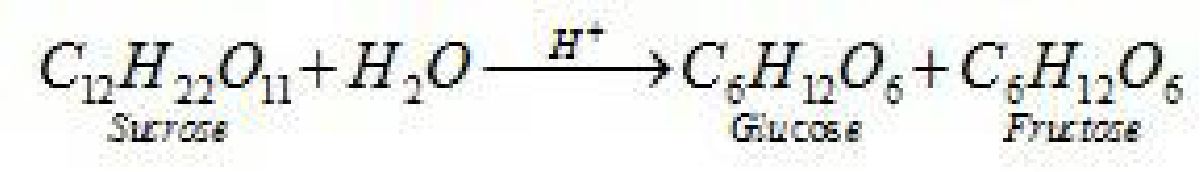
• From starch

• Structure of glucose
• Structure elucidation of glucose:
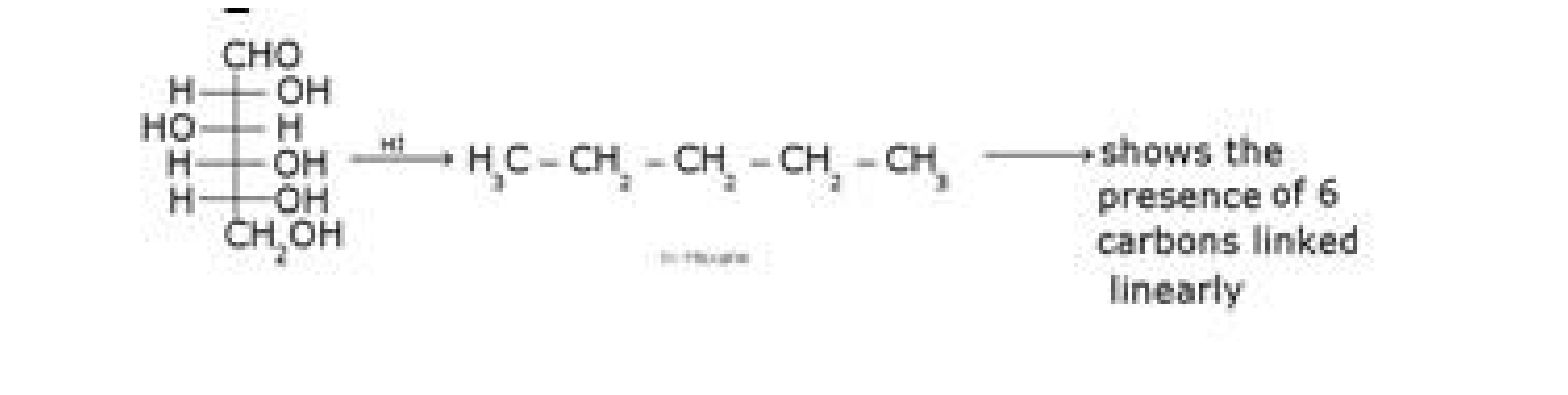
- D – glucose with HI
b) D – glucose with HCN
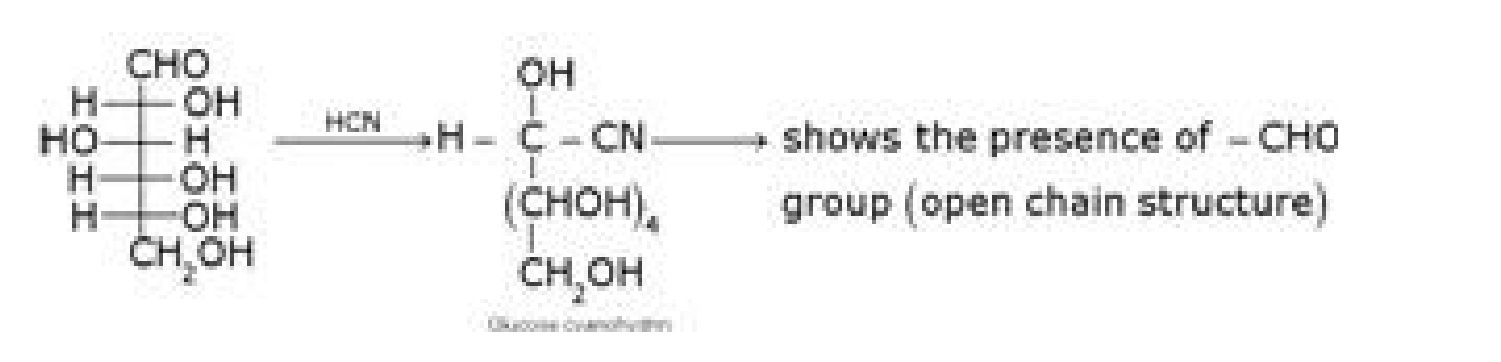
c) D – glucose with NH2OH

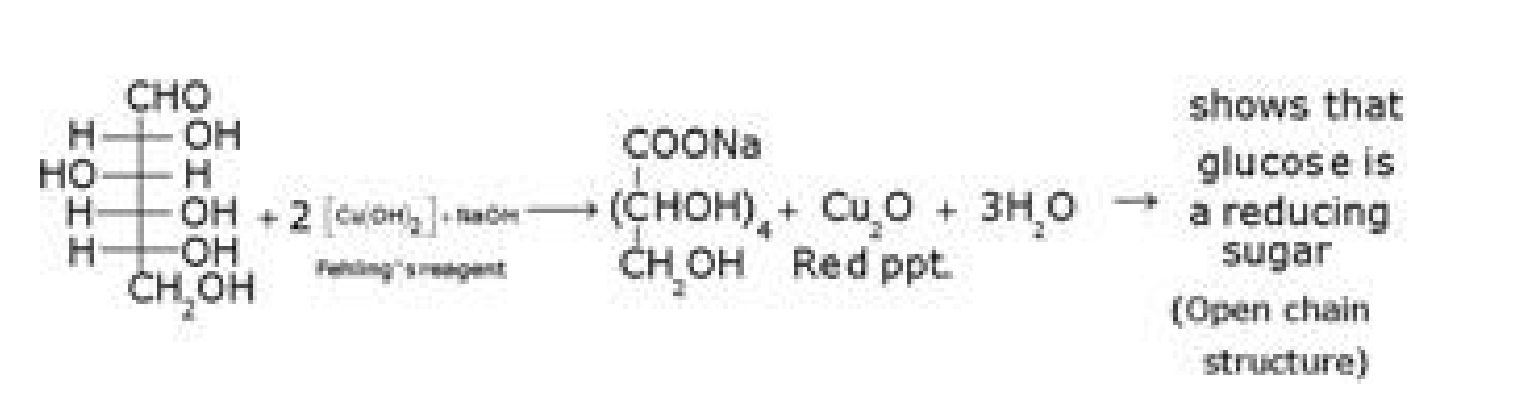
d) D- glucose with Fehling’s reagent
/ 15
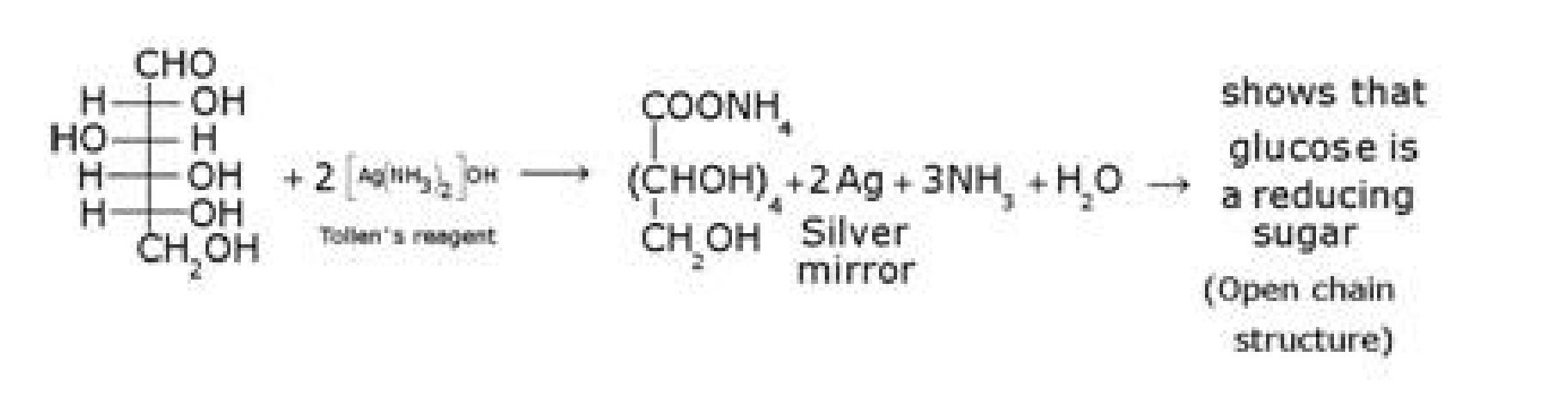
e) D – glucose with Tollen’s reagent
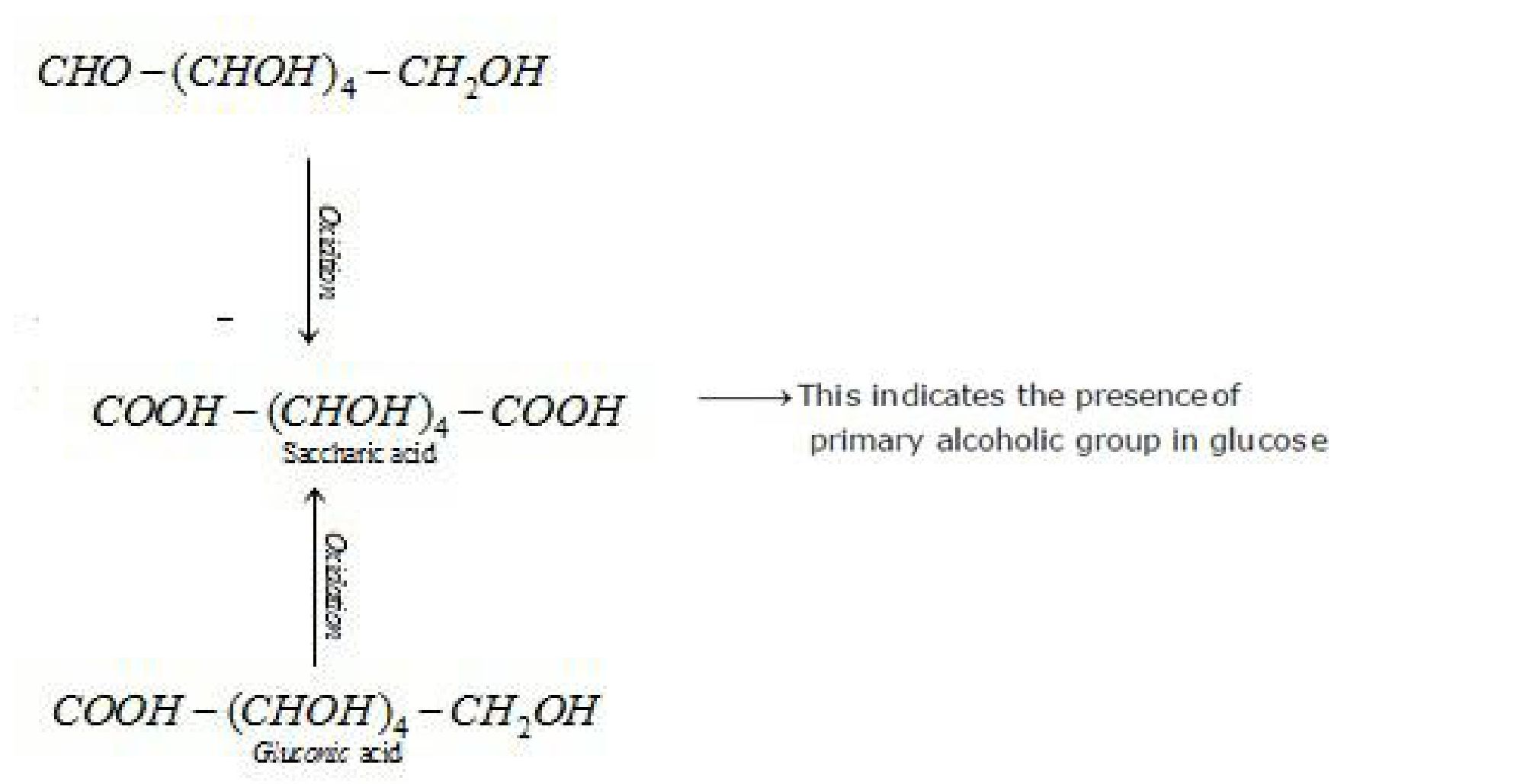
f) D – glucose with nitric acid
- D – glucose with (CH3CO)2O and ZnCl2
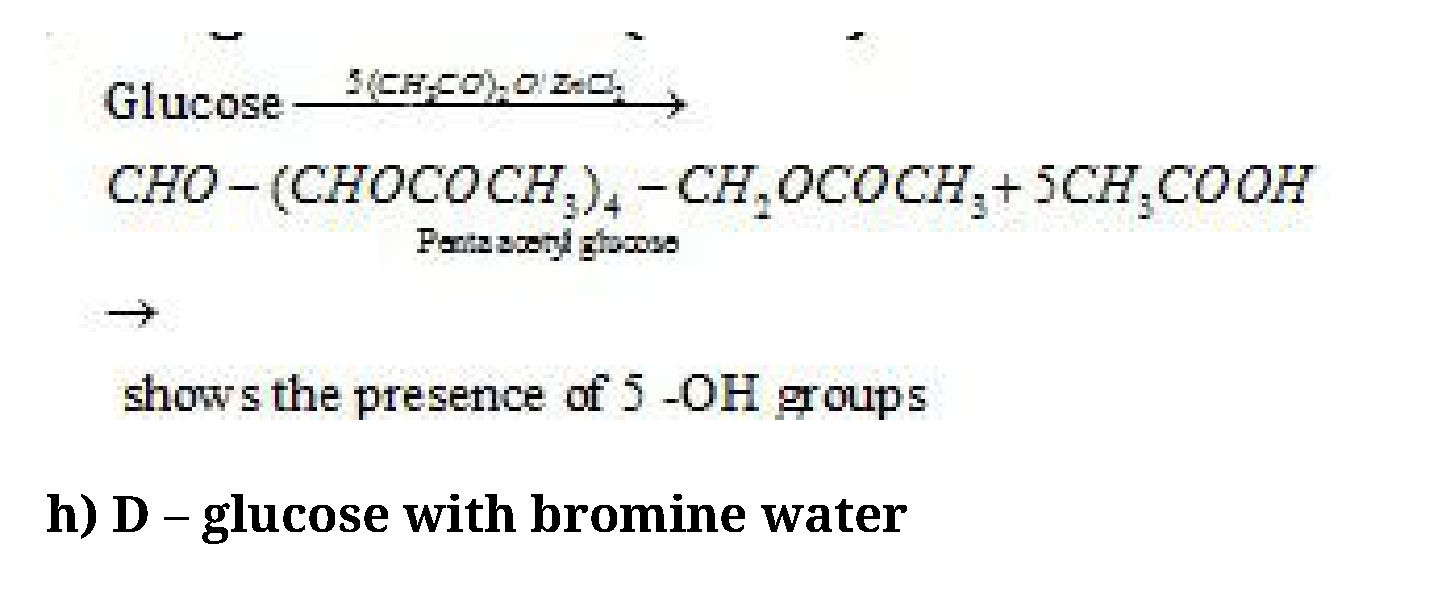
/ 15
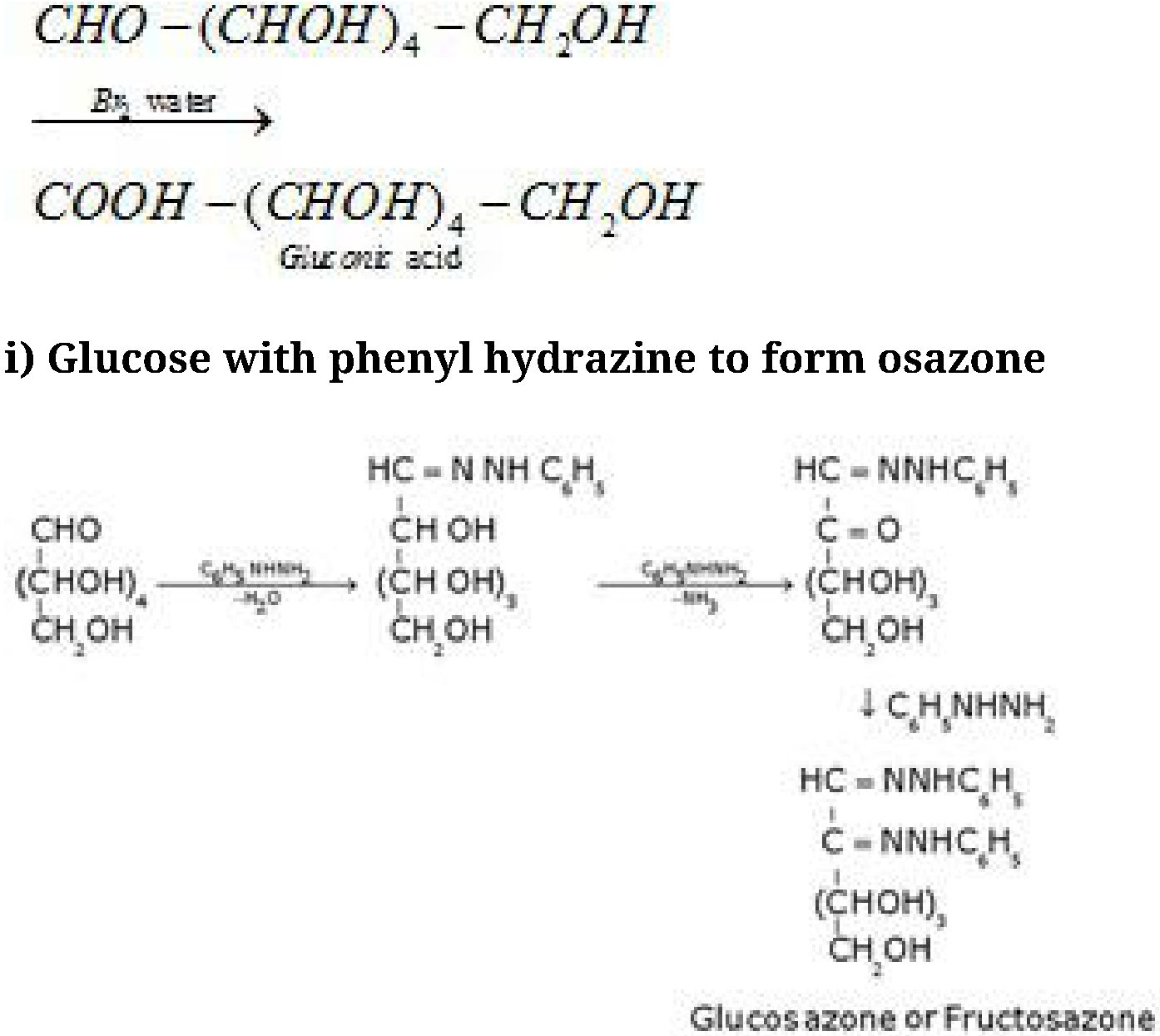
Glucose and fructose gives the same osazone because the reaction takes place at C1 and C2
only.
Other Reactions of Glucose (Presence of ring structure)
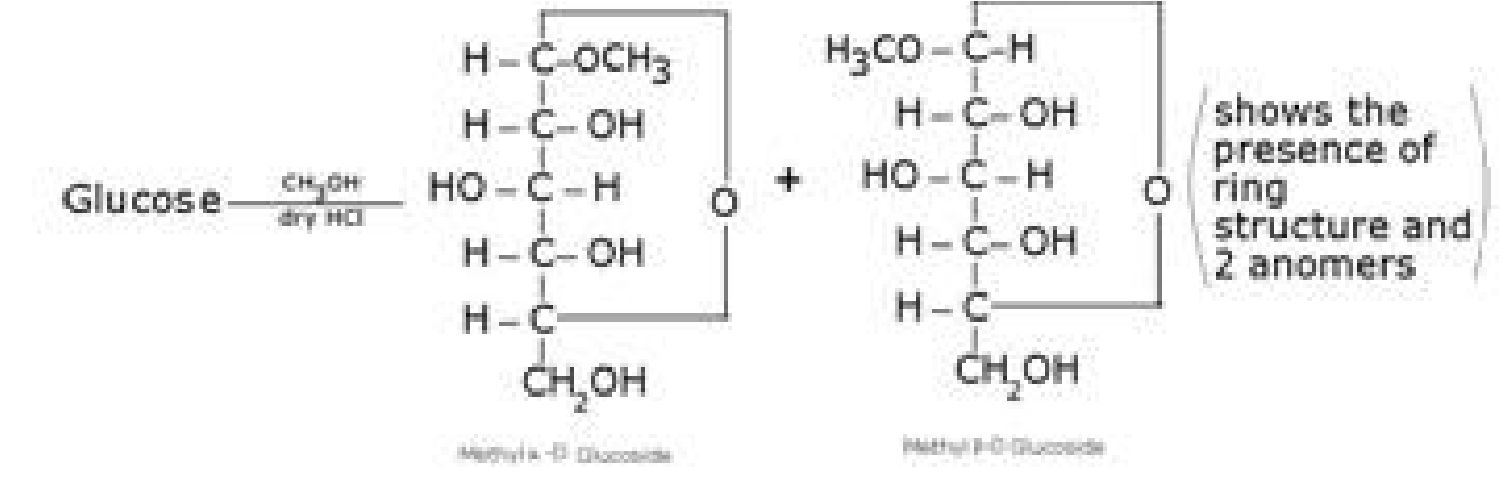
Glucose does not give Schiffs test and does not react with sodium bisulphite and NH3.
Pentaacetyl glucose does not react with hydroxyl amine. This shows the absence of -CHO
group and hence the presence of ring structure.

• Haworth representation of glucose:
Cyclic structure of fructose:
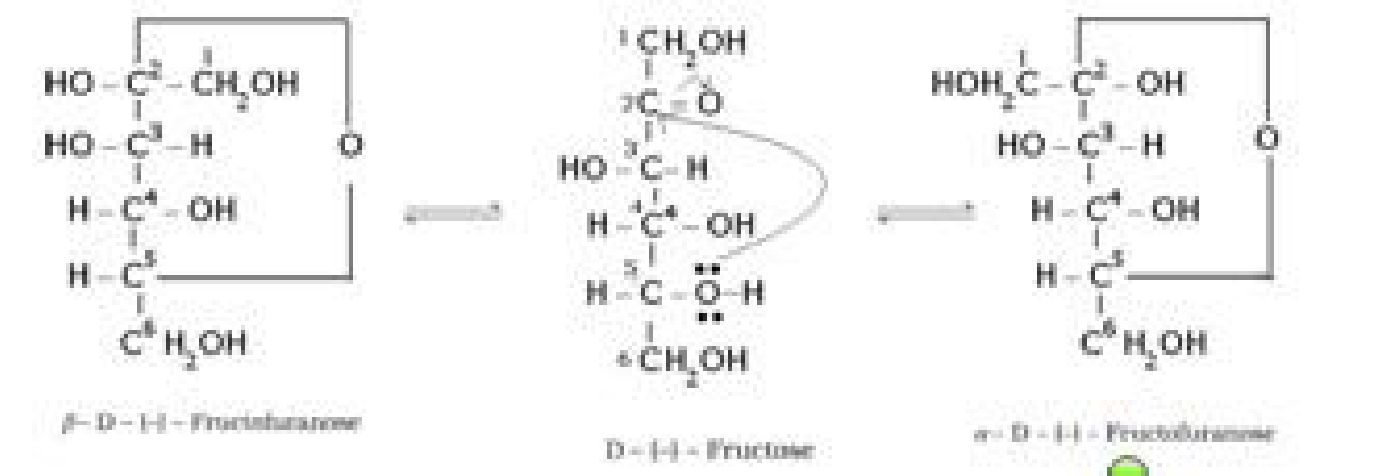
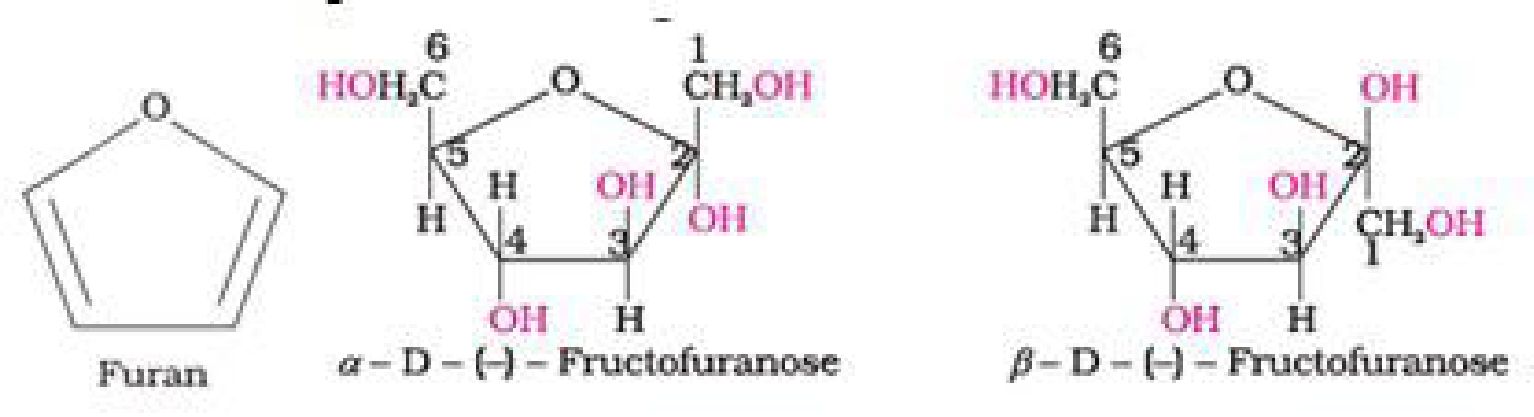
• Haworth representation of fructose
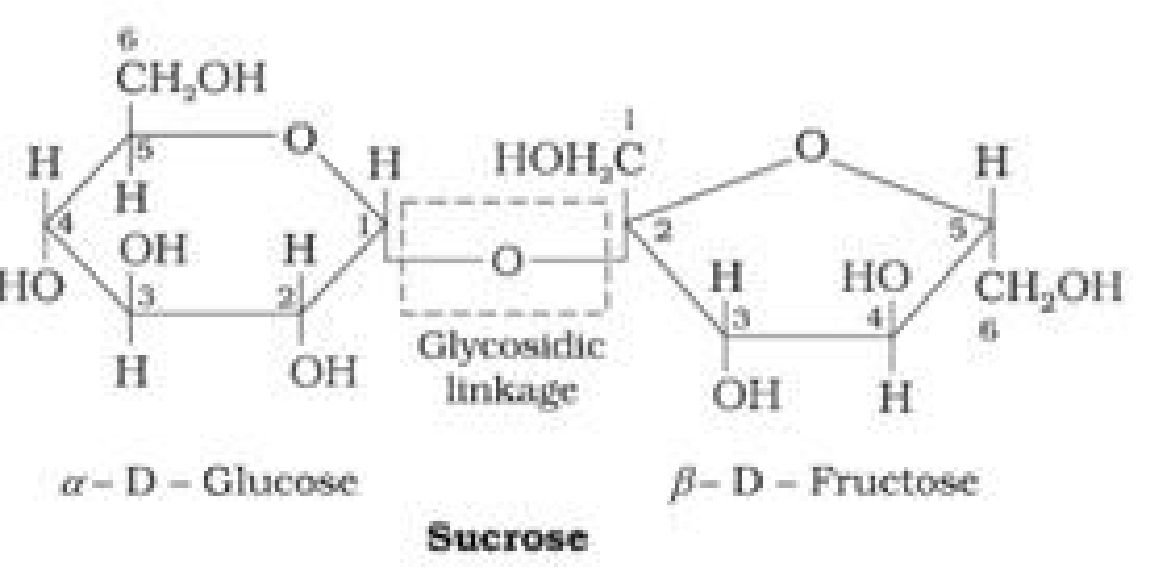
- Glycosidic linkage: The oxide linkage formed by the loss of a water molecule when
two monosaccharides are joined together through oxygen atom is called glycosidic
linkage. [1]
- Sucrose is a non-reducing sugar because the two monosaccharide units are held together
by a glycosidic linkage between C1 of ct-glucose and C2 of /3- fructose. Since the reducing
groups of glucose and fructose are involved in glycosidic bond formation, sucrose is a non-
reducing sugar.
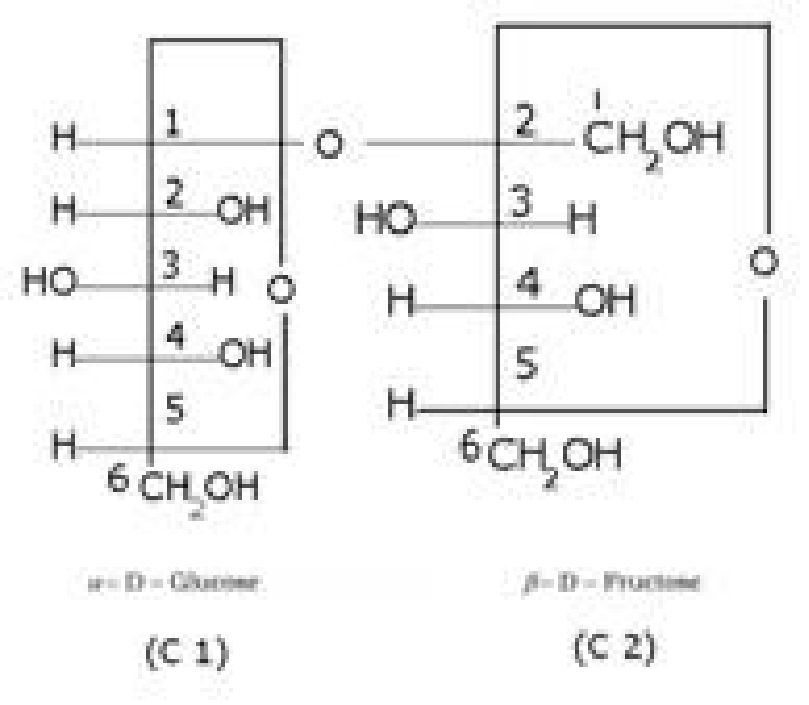
- Sucrose is dextrorotatory but on hydrolysis it gives dextrorotatory & laevorotatory and the
mixture is laevorotatory.
^CiHu°i+C‘Ru0–
D-%izcon D- r>urtare
[*.;.,-+:V^ IafcrJH^
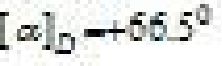
• Haworth Projection of Sucrose:

• Haworth projection of maltose:

• Lactose (Milk sugar):It is composed of p-D-galactose and p-D-glucose. The linkage is
between C1 of galactose and C4 of glucose. Hence it is also a reducing sugar.
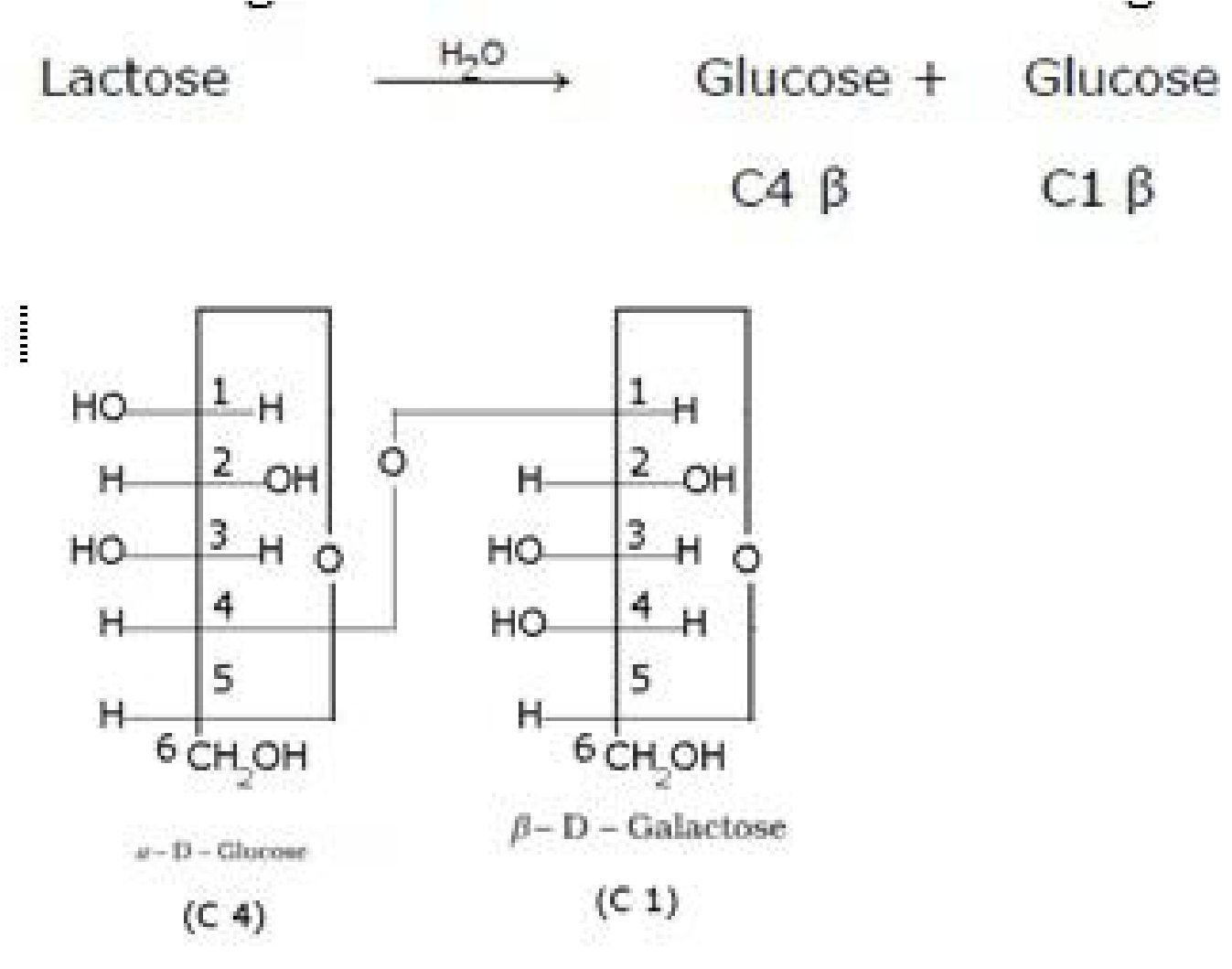
• Haworth projection of lactose:

- Starch: It is a polymer of -glucose and consists of two components — Amylose and
Amylopectin. - Amylose:
- It is a water soluble component
- It is a long unbranched chain polymer
- It contains 200 – 1000 a-D-(+)- glucose units held by a- glycosidic linkages involving C1 –
C4glycosidic linkage - It constitutes about 15-20% of starch
- It is a water insoluble component
- It is branched chain polymer
- It forms chain by C1 – C4glycosidic linkage whereas branching occurs by C1 –
C6glycosidic linkage - It constitutes about 80-85% of starch
- It occurs exclusively in plants.
- It is a straight chain polysaccharide composed only of /3-D-glucose units which are joined
by glycosidic linkage between C1 of one glucose unit and C4 of the next glucose unit.
- The carbohydrates are stored in animal body as glycogen.
- It is also known as animal starch because its structure is similar to Amylopectin.
- It is present in liver, muscles and brain.
- When the body needs glucose, enzymes break the glycogen down to glucose.
Amino acids contain amino (-NH2) and carboxyl (-COOH) functional groups.
L
Where R – Any side chain
Most naturally occurring amino acids have L – Config.
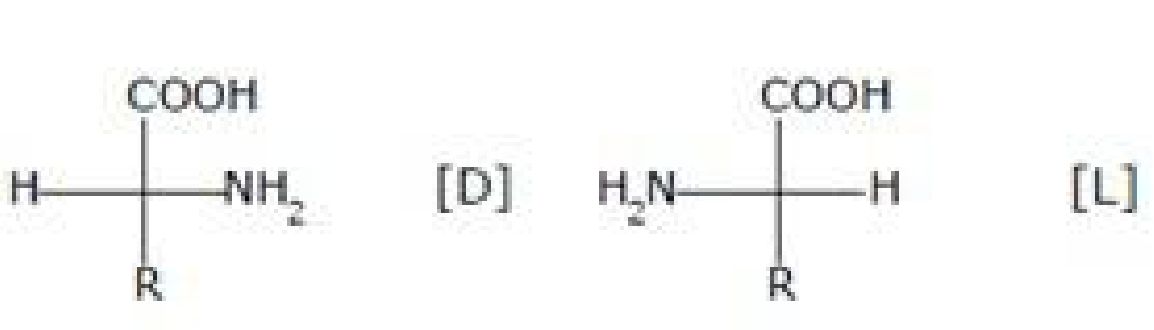
• Types of amino acids:
a). Essential amino acids: The amino acids which cannot be synthesised in the body and
must be obtained through diet, are known as essential amino acids. Examples: Valine,
Leucine
- . Non-essential amino acids: The amino acids, which can be synthesised in the body, are
known as non-essential amino acids. Examples: Glycine, Alanine
• Zwitter ion form of amino acids:
- Amino acids behave like salts rather than simple amines or carboxylic acids. This
behaviour is due to the presence of both acidic (carboxyl group) and basic (amino group)
groups in the same molecule. In aqueous solution, the carboxyl group can lose a proton
and amino group can accept a proton, giving rise to a dipolar ion known as zwitter ion.
This is neutral but contains both positive and negative charges. - In zwitter ionic form, amino acids show amphoteric behaviour as they react both with
acids and bases.
O O
R-CH-C-O-H^R- CH-C-O-
jffiR W1
2 j
■JSi iiii^ ioa^
- Isoelectronic point: The pH at which the dipolar ion exists as neutral ion and does
not migrate to either electrode cathode or anode is called isoelectronic point. - Proteins: Proteins are the polymers of a-amino acids and they are connected to each
other by peptide bond or peptide linkage. A polypeptide with more than hundred
amino acid residues, having molecular mass higher than 10,000u is called a protein. - Peptide linkage: Peptide linkage is an amide linkage formed by condensation
reaction between -COOH group of one amino acid and -NH2 group of another amino
acid.
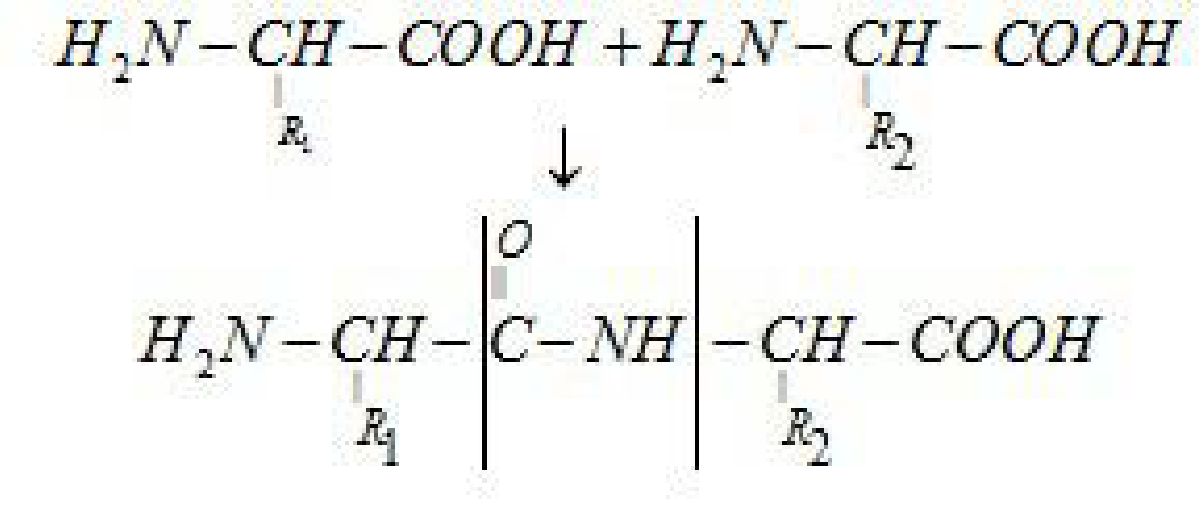
Peptide link age
- Primary structure of proteins: The sequence of amino acids is said to be the primary
structure of a protein. - Secondary structure of proteins: It refers to the shape in which long polypeptide
chain can exist. Two different types of structures:
a- Helix:
- It was given by Linus Pauling in 1951
- It exists when R- group is large.
- Right handed screw with the NH group of each amino acid residue H – bonded to – C = O
of adjacent turn of the helix. - Also known as 3.613 helix since each turn of the helix hasapproximately 3.6 amino acids
and a 13 – membered ring is formed by H – bonding.
- C = O and N – H group of the peptide bonds are trans to each other.
- Ramchandran angles ($and^) – $angle which C^makes with N – H and Wangle which
(7amakes with C = O.
/3- pleated sheet:
- It exists when R group is small.
- In this conformation, all peptide chains are stretched out to nearly maximum extension
and then laid side by side which are held together by hydrogen bonds.
- Tertiary structure of proteins: It represents the overall folding of the polypeptide
chain i.e., further folding of the 2° structure. - Types of bonding which stabilize the 3° structure:
- Disulphide bridge (-S – S-)
- H – bonding – (C = O … H – N)
- Salt bridge (COO- … + NH^)
- Hydrophobic interactions
- van der Waals forces
- When the polypeptide chains run parallel and are held together by hydrogen and
disulphide bonds, then fibre- like structure is formed. - These proteins are generally insoluble in water
- Examples: keratin (present in hair, wool, silk) and myosin (present in muscles), etc
Globular proteins - This structure results when the chains of polypeptides coil around to give a spherical
shape. - These are usually soluble in water.
- Examples: Insulin and albumins
- Some of the proteins are composedof two or more polypeptide chains referred to as sub-
units. - The spatial arrangement of these subunits with respect to each other is known as
quaternary structure of proteins.
- The loss of biological activity of proteins when a protein in its native form, is subjected to
physical change like change in temperature or chemical change like change in pH. This is
called denaturation of protein. - Example: coagulation of egg white on boiling, curdling of milk.
1. Base + sugar
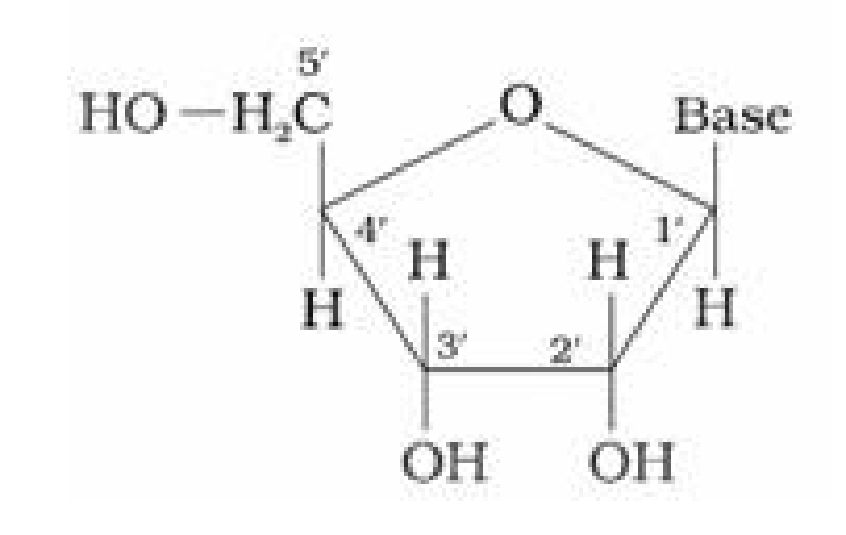
1. Base + sugar + phosphate group

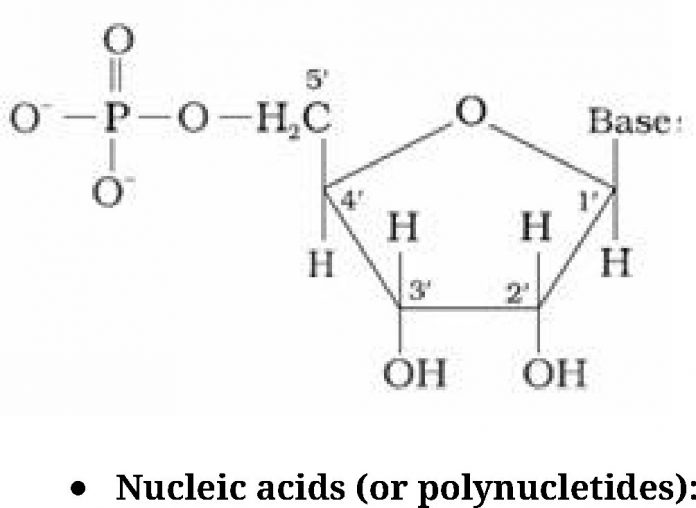
- Long chain polymers ofnucleotides.
- Nucleotides are joined by phosphodiester linkage between 5’ and 3’ C atoms of a pentose
sugar.
- It has a double stranded ct-helix structure in which two strands are coiled spirally in
opposite directions. - Sugar present is /3-D-2-deoxyribose
- Bases:
- Purine bases: Adenine (A) and Guanine (G)
- Pyrimidine bases: Thymine (T) and cytosine (C)
- It occurs mainly in the nucleus of the cell.
- It is responsible for transmission for heredity character.
- It has a single stranded a-helix structure.
- Sugar present is /3-D-ribose
- Bases:
- Purine bases: Adenine (A) and Guanine (G)
- Pyrimidine bases: Uracil (U) and cytosine (C)
- It occurs mainly in the cytoplasm of the cell.
- It helps in protein synthesis.
- It is composed of two right handed helical polynucleotide chains coiled spirally in
opposite directions around the same central axis. - Two strands are anti-parallel i.e., their phosphodiester linkage runs in opposite
directions. - Bases are stacked inside the helix in planes _Lto the helical axis.
- Two strands are held together by H – bonds (A = T, G =C).
- The two strands are complementary to each other because the hydrogen bonds are
formed between specific pairs of bases.
- Adenine forms hydrogen bonds with thymine whereas cytosine forms hydrogen bonds
with guanine. - Diameter of double helix is 2 nm.
- Double helix repeats at intervals of 3.4 nm. (One complete turn)
- Total amount of purine (A + G) = Total amount of pyramidine (C + T)
- Vitamins: Vitamins are organic compounds required in the diet in small amounts to
perform specific biological functions for normal maintenance of optimum growth and
health of the organism. - Classification of vitamins: Vitamins are classified into two groups depending upon
their solubility in water or fat.
- Water soluble vitamins
- These vitamins are soluble in water.
- Water soluble vitamins must be supplied regularly in diet because they are readily
excreted in urine and cannot be stored (except vitamin B12) in our body. - Example: Vitamin C, B group vitamins.
- Fat soluble vitamins
- These vitamins are soluble in fat and oils but insoluble in water.
- They are stored in liver and adipose (fat storing) tissues.
- Example: Vitamin A, D, E and K
• Important vitamins, their sources and their deficiency diseases:
|
Name of |
Sources |
Deficiency diseases |
|
Vitamin A |
Fish liver oil, |
xerophthalmia |
|
Vitamin B1 |
Yeast, milk, green |
Beriberi (loss of appetite, retarded growth) |
|
Vitamin B2 |
Milk, egg white, liver, |
Cheilosis (fissuring at corners of mouth and lips), digestive |
|
Vitamin B6 |
Yeast, milk, egg yolk, |
Convulsions |
|
Vitamin B12 |
Meat, fish, egg and curd |
Pernicious anaemia (RBC deficient in haemoglobin) |
|
Vitamin C |
Citrus fruits, amla and |
Scurvy (bleeding gums) |
|
Vitamin D |
Exposure to sunlight, fish |
Rickets (bone deformities in children) and (soft bones and joint pain in adults) |
|
Vitamin E |
Vegetable oils like wheat |
Increased fragility of RBCs and |
|
Vitamin K |
Green leafy vegetables |
Increased blood clotting time |
• Maltose:
Maltose is composed of two a-D-glucose units in which C1 of one glucose (I) is linked to C4
of another glucose unit (II).
The free aldehyde group can be produced at C1 of second glucose in solution and it shows
reducing properties so it is a reducing sugar.
-
Sucrose (invert sugar): ↑