CBSE Class-12 Chemistry
Quick Revision Notes
Chapter 15
Polymers
- Polymers: Polymers are high molecular mass substance consisting of large number of
repeating structural units. As polymers are single, giant molecules i.e. big size
molecules, they are also called macromolecules - Monomers: The simple molecules which combine to form polymers by forming single
or multiple bonds are called monomers. - Polymerization: The process of formation of polymers from respective monomers is
called polymerization - Classification of Polymers:
- Based on source of availability, it is classified into
- Natural polymers: Polymers obtained from nature, mostly plants and animals.
Examples – Cellulose, starch, etc. - Synthetic polymers: Polymers prepared in laboratory. Examples – Teflon, Nylon 6,6 ,
Synthetic rubber (Buna – S) etc. - Semi synthetic polymers: Polymers derived from naturally occurring polymers by
carrying out chemical modifications. Examples – Rayon (cellulose acetate), cellulose
nitrate, etc. - Based on the structure of polymer, it is classified into
- Linear polymers: Polymer consists of long and straight chains. Examples – High
density polythene, polyvinyl chloride, etc. - Branched chain polymers: Polymers contains linear chains having some branches.
Examples – Low density polythene - Cross linked or network polymers: Polymers in which monomer units are cross linked
together to form a 3 dimensional network polymers. Examples – Bakelite, melamine,
etc. - Based on the mode of polymerisation, it is classified into
- Addition polymers: Polymers are formed by the repeated addition of monomers with
double and triple bonds. It is further classified into,
Homopolymers:Polymers formed by the polymerisation of a single monomeric species.
Examples – Polythene, Polystyrene.
Copolymers:Polymers formed by addition polymerisation of two different monomers.
Examples – Buna-S, Buna -N.
- Condensation polymers: Polymers formed by repeated condensation reaction between
two different bi-functional or tri-functional monomeric units with elimination of simple
molecules. Examples – Nylon 6, 6, Nylon 6.
Based on Molecular forces, it is classified into
Step 1: Chain initiating step: Organic peroxides undergo homolytic fission to form free
radicals which acts as initiator. Initiator adds to C-C double bond of an alkene molecule to
form a new free radical
O O O
Ii fus I il . *
<VJ,^KW>C-Crt >2C^>0^-2CA +2C0,
Bcnznyi pmaKk ETwmi redjcaJ
* ■
C*H*+CHj=CKt * CJi,-CJi:-CHL
Step 2: Chain propagating step: Free radicals formed by homolytic cleavage adds to a double
bond of monomer to form a larger free radical. Radical formed adds to another alkene
molecule to form a larger free radical. This process continues until the radical is destroyed.
These steps are called propagation steps.
CiHi ~ CH1 – C H1 – CH1 = CH1
CJii – CH1 – CH1 – CH1 – C H1
¥
CiHi – CCH1 – CH^ – CH1 – C H1
Step 3: Chain terminating step: For termination of the long chain, free radicals combine in
different ways to form polythene. One mode of termination of chain is shown as under:
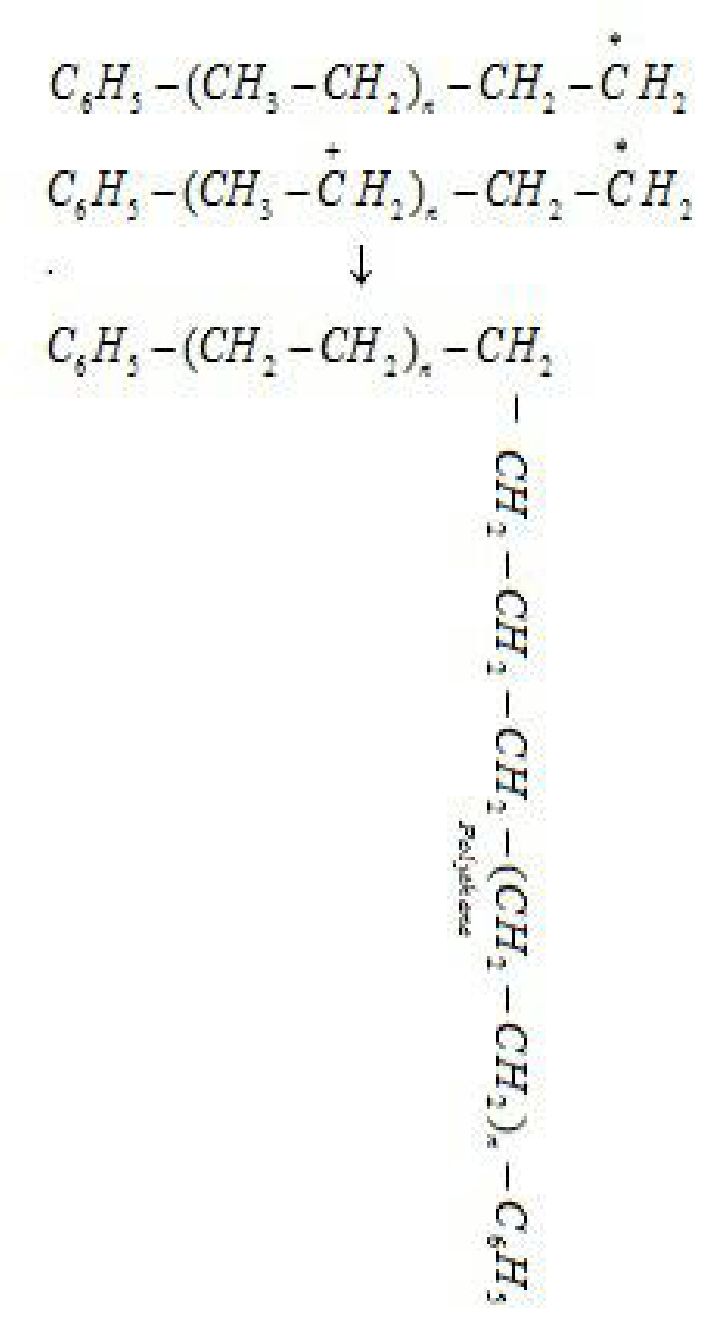
- . Low density polythene (LDP) is a polymer of ethene.
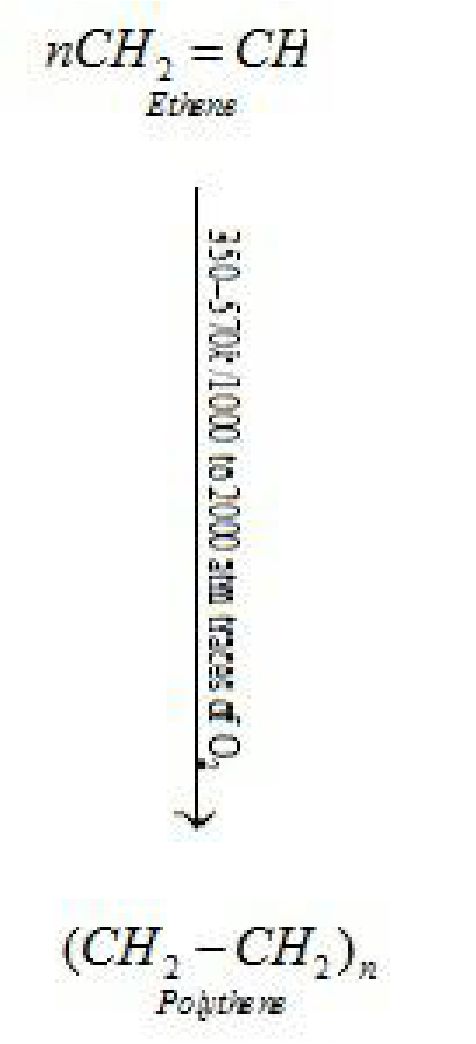
It is used in the insulation of electricity carrying wires and manufacture of squeeze bottles,
toys and flexible pipes
- . High density polythene(HDP) is a polymer of ethene.

It is used for manufacturing buckets, dustbins, bottles, pipes, etc.
- . Polytetrafluoroethene (is a polymer of Teflon)
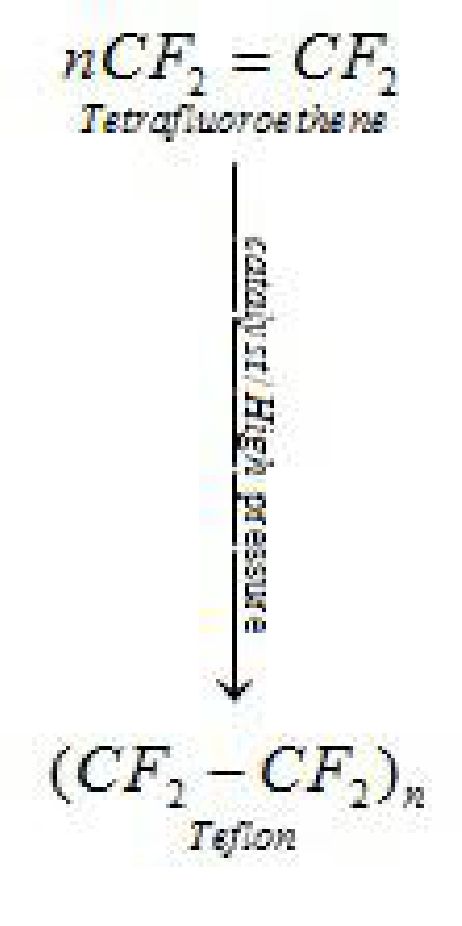
It is used in making oil seals and gaskets and also used for non – stick surface coated utensils
- . Polyacrylonitrile is a polymer of acrylonitrile.
It is used as a substitute for wool in making commercial fibres such as orlon or acrilan.
1. Polyamides: Polymers possess amide linkage (-CONH-) in chain. Thesepolymers are
popularly known as nylons. Examples:
(a) Nylon 6, 6: It is prepared by the condensation polymerisation of hexamethylenediamine
with adipic acid under high pressure and at high temperature.
KHOOC(CH2)4COOH+ }TH2H(CH:)^XH2
It is used in making sheets, bristles for brushes and in textile industry.


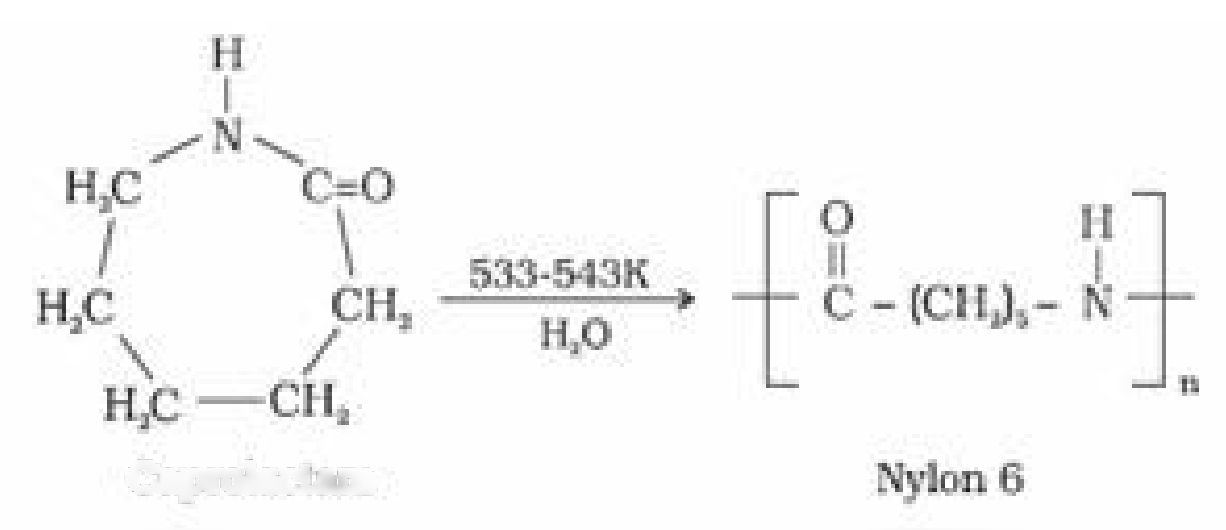
(b) Nylon 6: It is obtained by heating caprolactum with water at a high temperature
CupraUcLam
It is used for the manufacture of tyre cords, fabrics and ropes.
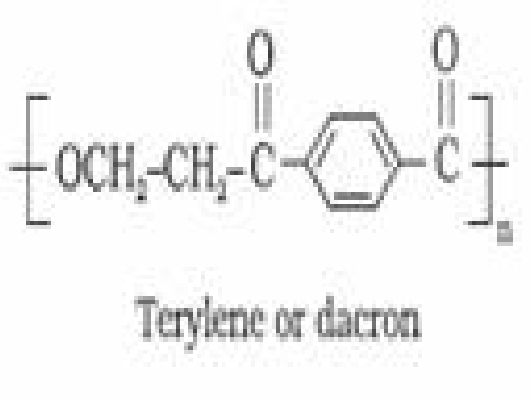
- Polyesters: These are the polycondensation products of dicarboxylic acids and diols
Example: Terylene or Dacron
n HOHt- CH1OH t n HOOCn0- COOH —*
Efl^DK^ol TerepbLhabc ^rid
|Etha^!.2’dKfl (BmrKie-!.4 – dl
It is used to create resistance in polymerised product and is used in blending with cotton and
wool fibres and also as glass reinforcing materials in safety helmets, etc.
- Phenol – formaldehyde polymer (Bakelite and related polymers)
a). Bakelite: These are obtained by the condensation reaction of phenol with formaldehyde
in the presence of either an acid or a base catalyst. The initial product could be a linear
product – Novolac used in paints.

b). Novolac on heating with formaldehyde forms Bakelite
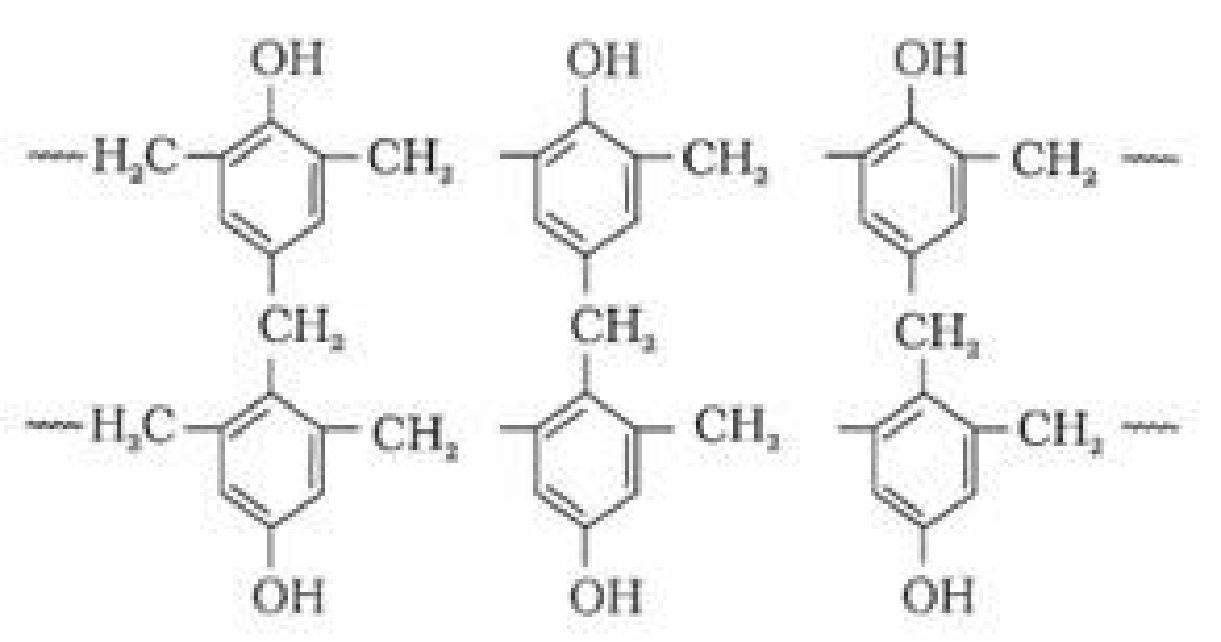
It is used for making combs, phonograph records, electrical switches and handles of various
utensils
- Melamine – formaldehyde polymer: Melamine formaldehyde polymer isformed by the
condensation polymerisation of melamine and formaldehyde

It is used in the manufacture of unbreakable crockery.

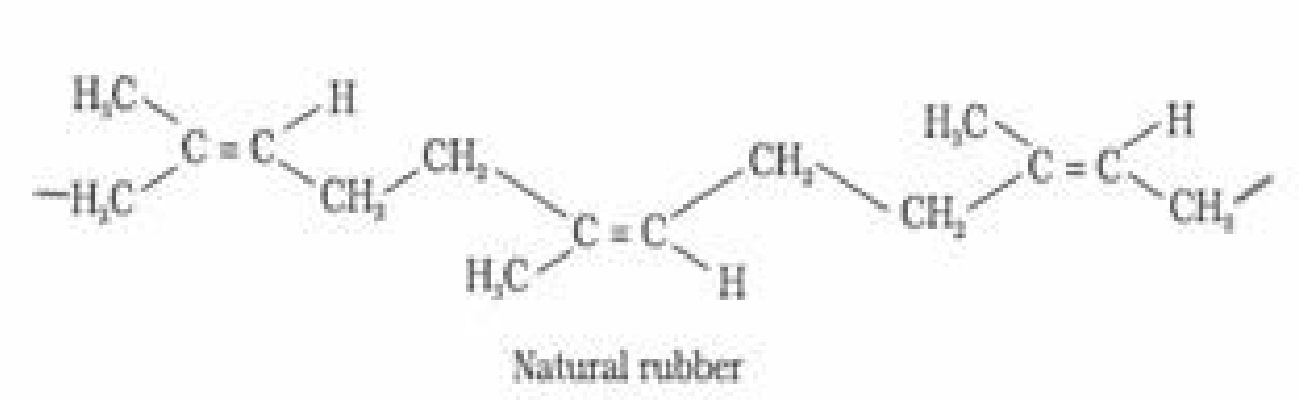
a). Natural rubber: Natural rubber is a linear polymer of isoprene (2-methyl-1, 3-butadiene)
and is also called as cis – 1, 4 – polyisoprene.
b). Synthetic rubber: Synthetic rubbers are either homopolymers of 1, 3 – butadiene
derivatives or copolymers of 1, 3 – butadiene or its derivatives with another unsaturated
monomer.

It is used for manufacturing conveyor belts, gaskets and hoses
B) Buna – N

It is used in making oil seals, tank lining, etc. because it is resistant to the action of petrol,
lubricating oil and organic solvents
C) Buna – S



It is used in speciality packaging, orthopaedic devices and in controlled release of drugs.
b). Nylon 2-nylon 6: It is an alternating polyamide copolymer of glycine(H2N-CH2-COOH)
and amino caproic acid (H2N (CH2)5 COOH)
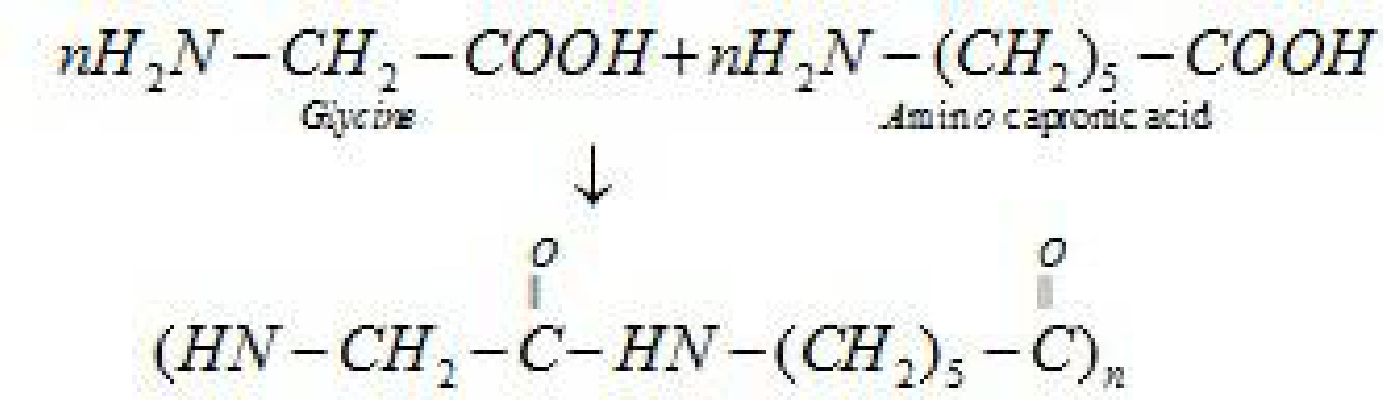
- Elastomers: Polymer chains are held together by weakest intermolecular forces.
Polymers are rubber – like solids with elastic properties. Examples – Buna – S, Buna – N,
Neoprene. - Fibre: Polymers have strong intermolecular force like hydrogen bonding. Fibres are the
thread forming solids which possess high tensile strength and high modulus. Examples –
Nylon 6, 6, Polyesters. - Thermoplastic polymers: Polymers are held by intermolecular forces which are in
between those of elastomers and fibres. These polymers are capable of repeated
softening on heating and hardening on cooling. Examples – Polythene, Polystyrene. - Thermosetting polymers: Polymers are cross linked or heavily branched molecules,
which on heating undergo extensive cross linking in moulds and eventually undergo a
permanent change. Examples – Bakelite, Urea-formaldelyde resins
- Addition Polymerisation or Chain Growth Polymerisation: Addition polymerisation is
called chain growth polymerisation because it takes place through stages leading to
increase in chain length and each stage produces reactive intermediates for use in next
stage of the growth of chain. Most common mechanism for addition polymerisation
reactions is free radical mechanism
Condensation Polymerisation or Step Growth polymerization: Polymerisation generally
involves a repetitive condensation reaction between two bi-functional monomers. In
condensation reactions, the product of each step is again a bi-functional species and the
sequence of condensation goes on. Since, each step produces a distinct functionalized species
and is independent of each other, this process is also called as step growth polymerisation.
Terylene or Dacron: It is manufactured by heating a mixture of ethylene glycol and
terephthalic acid at 420 to 460 K in the presence of zinc acetate-antimony trioxide catalyst.
Vulcanisation of rubber: The process of heating a mixture of raw rubber with sulphur and
an appropriate additive in a temperature range between 373 K to 415 K to improve upon
physical properties like elasticity, strength etc.
Biodegradable Polymers: Polymers which are degraded by microorganisms within a suitable
period so that biodegradable polymers and their degraded products do not cause any serious
effects on environment.
Examples of biodegradable polymer:
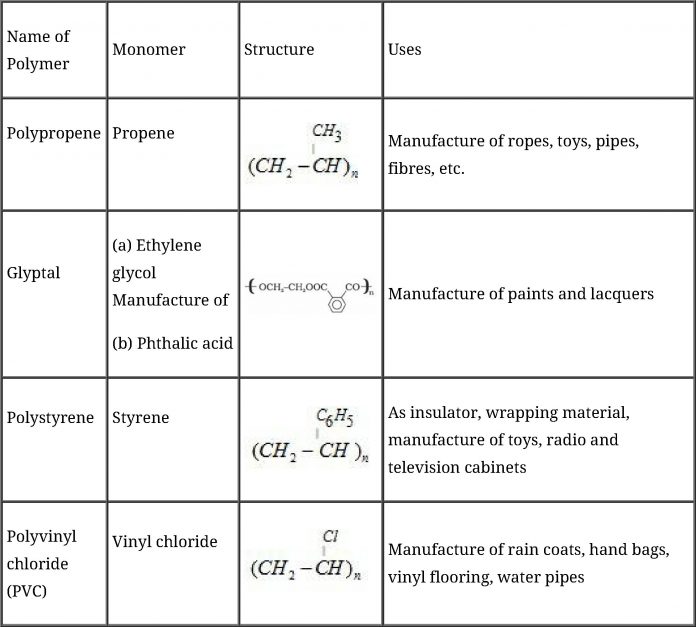
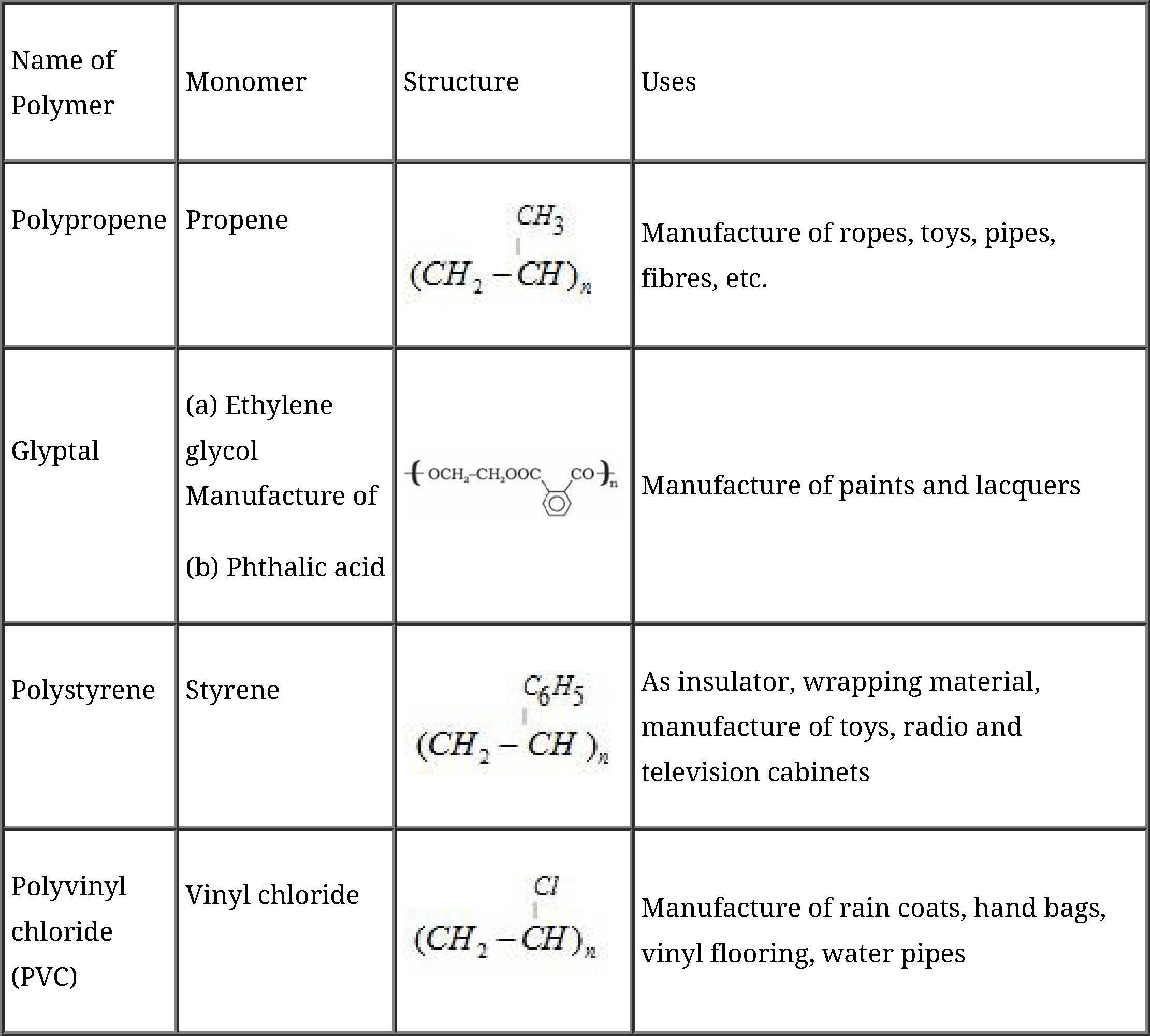
Commercially important polymers along with their structures and uses: